

Using PDX Models for translational research, shows how we can overcome resistance through combination therapies in Preclinical Breast Cancer HR+/HER2- Models.
Breast cancer is the most common cancer worldwide and the leading cause of cancer-related deaths among women. Approximately 70-80% of breast cancers are classified as HR+/HER2–, so-called luminal A breast cancers. Initially, HR+/HER2– patients are treated with hormone therapy (HT) including antiestrogens that interfere with the ER pathway (e.g., tamoxifen or fulvestrant) or estrogen deprivation strategies including aromatase inhibitors or ovariectomy. Unfortunately, most patients develop HT resistance, with chemotherapy being the only remaining option.
Addressing Resistance in HR+/HER2- Breast Cancer with CDK 4/6 Inhibitors
The recent development of CDK 4/6 inhibitors, such as palbociclib, ribociclib and abemaciclib significantly changed treatment options for HR+/HER2- patients with HT resistance, pushing chemotherapy back into later lines. However, many of those patients eventually develop resistance to CDK 4/6 inhibitor containing regimens, raising the question of what the next best treatment option is, pure chemotherapy or adding chemotherapy to existing CDK 4/6 inhibitor-containing regimens.
Exploring Eribulin’s Potential – Bridging Cytotoxic and Biology-Modifying Actions for Synergistic Combination Therapies in Breast Cancer
As a microtubule dynamics inhibitor, eribulin* exerts cytotoxic antimitotic activities that are typical of most tubulin-targeted agents. In addition, eribulin’s non-cytotoxic tumor biology-modifying effects support the hypothesis that it interacts with additional pathways. Such additional activities suggest that eribulin may be a good candidate to combine with other drugs acting via different mechanisms, especially those exerting effects at the G1/S cell cycle transition point where proliferation and cellular differentiation signaling pathways converge.
*Eribulin, a macrocyclic ketone analog of the marine sponge natural product halichondrin B has been approved for the treatment of patients with advanced breast cancer, advanced liposarcoma, or soft tissue sarcoma. In addition to the cytotoxic antimitotic, eribulin also affects tumor vasculature leading to increased tumor perfusion and mitigation of tumor hypoxia, reversal of epithelial-mesenchymal transition in breast cancer and cell differentiation in sarcoma, and therapeutically beneficial alterations of the tumor immune microenvironment including increased post-treatment CD8 T cells in patient tumor samples and activation of innate immune signaling.
Distinct Antitumor Effects of Drug Doublet and Triplet Combination Revealed in Experimental Study
We previously observed synergistic antitumor effects between eribulin and the CDK 4/6 inhibitor palbociclib in two PDX models of HR+/HER2- luminal breast cancer. We next extended exploration of eribulin plus palbociclib in vivo synergy by introducing the fulvestrant to directly inhibit ER pathway activity and mimic closer the clinical situation of patients treated with hormone therapy and palbociclib.
Using the OD-BRE-0192 PDX model, doublet and triplet combinations of eribulin, palbociclib and fulvestrant were tested. At the tested doses, monotherapy of the three drugs led to only modest inhibition of tumor growth rates. In contrast, all three doublet combinations led to tumor stasis during the administration period, followed by a three-week delay before tumor growth resumed. Based on tumor regrowth rates, the two fulvestrant-containing doublets were the most effective. In contrast to the stasis achieved with the three doublets, the triplet combination of eribulin + palbociclib + fulvestrant (in blue on figure) led to marked tumor regression during the treatment period, followed by post-treatment persistence of stasis until the end of the study.
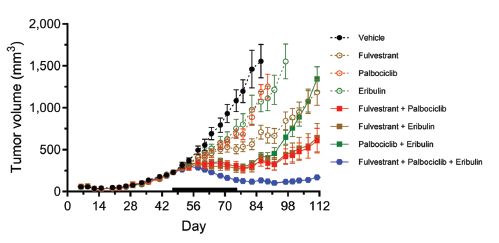
In vivo antitumor activity of eribulin, palbociclib and fulvestrant combinations in the OD-BRE-0192 breast cancer PDX model. Dosing schedule showing 0.25 mg/kg eribulin given intravenously (iv) on Q7D weekly cycles, 150 mg/kg palbociclib given orally (po) on Q5D weekly cycles and 0.625 mg/kg fulvestrant given subcutaneously (sc) on Q7D weekly cycles. Data are presented as means±SEM, with the day 47-73 treatment period indicated by solid bars on the x-axes.
Regarding best response by mRECIST criteria*. Nine of ten mice in the triplet combination group achieved PR status, with the tenth mouse achieving SD; thus, 100% of the mice in the triplet group benefitted and showed some degree of disease control.
*RECIST criteria (=Response Evaluation Criteria In Solid Tumors) is a set of published rules that define when tumors in cancer patient improve (“Response” PR), stay the same (“Stabilize” SD) or worsen (“Progress” PD) during treatment. The modified RECIST criteria only measure the enhanced portion of lesion.
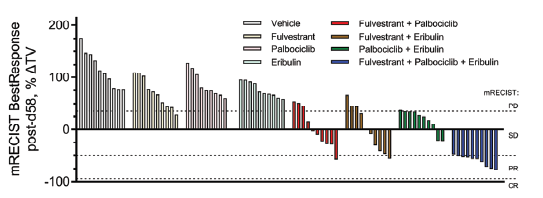
Best response categorization as progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR) using mRECIST criteria adapted for murine tumor studies.
Triple Combination of Eribulin, Fulvestrant, and Palbociclib Demonstrates Superior Tumor Regression in HR+ Breast Cancers
All three doublets led to identical stasis during the last three weeks of dosing, indicating that regardless of mechanism, combining any two of the three drugs is sufficient to fully halt tumor proliferation, yet without tumor regression. Interestingly, 2-3 weeks after dosing cessation, tumor regrowth in the two fulvestrant-containing doublets resumed slowly while regrowth in the eribulin + palbociclib group accelerated rapidly, suggesting that fulvestrant exerts long-lasting post-treatment effects. In contrast, clear and persistent tumor regression was observed in the triple combination group starting only one week after initiation of dosing, ultimately resulting in very small tumors that remained static for the remainder of the experiment.
As palbociclib is approved in the context of HR+ breast cancers, we first showed that doublet combination with fulvestrant can lead to tumor stasis (similarly to doublet combination with eribulin) but, only the triplet combination of eribulin +fulvestrant + palbociclib can induce marked and long-lasting tumor regression.
This article is based on the scientific paper “Doublet and Triplet Combinations of Eribulin, Fulvestrant, and Palbociclib in Preclinical Breast Cancer Models” (ANTICANCER RESEARCH 44: 61-70 (2024) doi:10.21873/anticanres.16788), co-written by Ismahene Benzaid Manseur, Study & Research Director, Elodie Marie Dit Chatel, Study & Research Technician, Marc Hillairet De Boisferon, Head of In Vivo Pharmacology department from at Oncodesign Services, and Bruce A. Littlefield from Eisai Inc.


