

Reversible covalent binders and the potential of benzoxaboroles
Historically, the pharmaceutical industry largely tried to avoid drugs that form covalent bonds with their targets. The thought was that the risks of off-target effects were too great – if the drug binds irreversibly in the wrong place, any side-effects might affect the patient in the long-term. But thinking has changed, with several drugs that bind covalently already on the market, including Abbvie’s ibrutinib and AstraZeneca’s osimertinib. Both of these drugs have annual sales in the billions.
If this fear of covalent binding can be overcome, then there is huge potential to hit targets previously thought undruggable. A good example here is Amgen’s sotorasib, which inhibits KRAS(G12C), which had previously proved resistant to many years of drug discovery efforts.
What else might a covalent binder be able to target? And what new technologies might expand the range of targets available to covalent drugs?
The idea behind a covalent binder is to form a covalent bond between one of the amino acids within a protein and a small molecule that has an appropriate reactive group. If this amino acid is present at the target, then forming this bond with the drug should be able to shut down its activity. Efficacy can be better, lower doses might be possible, and as fewer doses are likely to be necessary, patient compliance may well improve.
Most covalent drug research efforts thus far have focused on binding to the amino acid cysteine in a target protein, as it contains a thiol group that is particularly reactive. But it is not the only amino acid that might be targeted by a covalent binder: several other amino acids contain nitrogen or oxygen atoms that could be used to form bonds with a drug molecule.
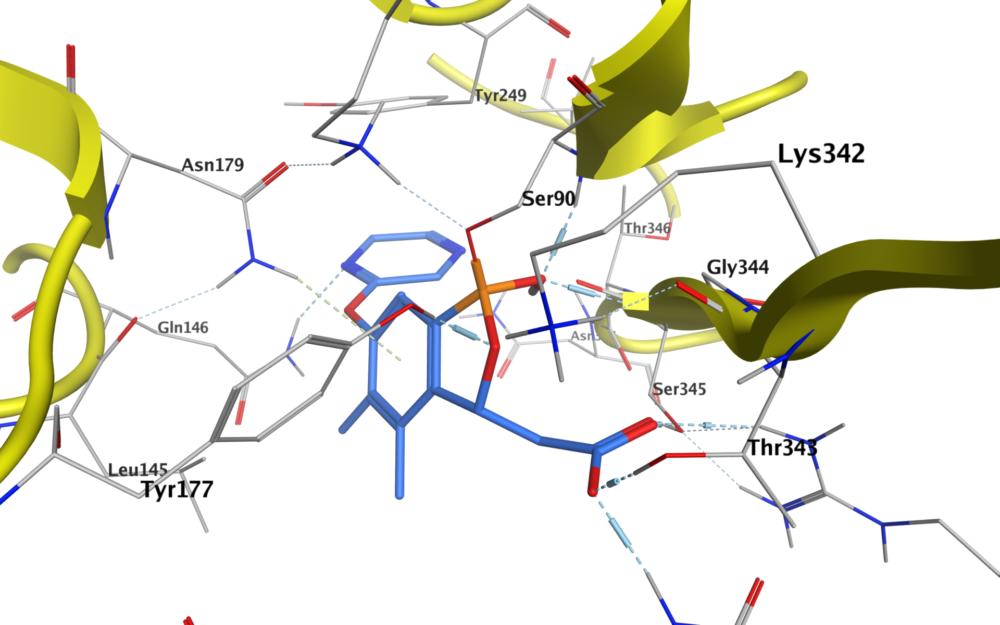
How might these heteroatoms atoms be targeted? One idea is to use drugs containing boron, as this can form reversible covalent bonds with the nucleophilic oxygen in serine, for example.
Boron may seem like an exotic choice, but a handful of boron-containing drugs are already on the market. These include Pfizer’s crisaborole, used to treat eczema, and tavaborole, an antifungal drug; two cancer treatments from Takeda, bortezomib and ixazomib; and Melinta Therapeutics’ vaborbactam, which prevents beta-lactam drugs from being broken down before they can have an antibacterial effect.
Molecules based on the benzoxaborole moiety are particularly interesting as potential drugs. These contain a boronic ester in a strained five-membered ring, which increases its Lewis acidity. This boron-containing group first bonds with the hydroxyl group in a serine residue, and the resulting stabilised complex can form ionic interaction or hydrogen bond with adjacent lysine or tyrosine residues.
The benzoxaborole group is also relatively stable. Molecules containing them typically have good solubility and permeability, two properties that are very advantageous in a drug. They also tend to be metabolically stable, reducing the chances of the drug being broken down in the body before it reaches its target.
Here at Oncodesign Services, we recognised the potential advantages of the benzoxaborole group, and our chemists designed and synthesised a library of benzoxaboroles that can be used in screening programmes. We now have nearly 300 compounds in this library that are already available for screening, at least 450 more that are ready to be synthesised, and a virtual library of almost 7000 more. Importantly, they fall into very different chemical space from existing libraries of covalent binders, greatly extending the potential for finding successful covalent drugs in the future.
To go deeper into the topic, read the full article. Please complete the form below :
About the author
This article was written by Christophe Parsy PhD, MBA, head of medicinal chemistry at Oncodesign Services. Christophe has more than 23 years of drug discovery experience. He holds a PhD in organic chemistry from the University of Liverpool, and an executive MBA from the Montpellier Business School.
Reference image :
- Article : https://pubmed.ncbi.nlm.nih.gov/27622821/
- Code PDB : 4WYY
