

On the onset of the lockdown, VALNEVA, a Franco-Austrian company specializing in the development, manufacturing, and marketing of prophylactic vaccines, engaged our services to assess the efficacy of the VLA 2001 vaccine against COVID-19. Collaborating with VALNEVA and the IMVA-HB/IDMIT research unit at CEA Paris-Saclay, Oncodesign Services conducted in vitro and in vivo pharmacology studies to evaluate this novel vaccine approach.

On April 3rd 2024, the scientific journal Communication Medicine published a study titled “Immunogenicity and Efficacy of the VLA2001 Vaccine Against SARS-CoV-2 Infection in Male Cynomolgus Macaques” (Commun Med 4, 62 (2024), https://doi.org/10.1038/s43856-024-00488-w). This study outlines a portion of the preclinical trials conducted on the VLA 2001 vaccine, now marketed by VALNEVA to combat COVID-19.
Throughout these studies, expert teams analyzed the immune responses elicited by the VLA2001 vaccine candidate in animal models, specifically with non human primate (NHP). These models aimed to demonstrate the vaccine’s ability to induce an immune response to impede virus spread and eliminate infected cells
Oncodesign Services contributed at two key stages:
- Verification of the immunogenicity of the VLA 2001 adjuvanted vaccine:
The primary objective was to confirm the antibody response to the vaccine. Following initial vaccination and booster doses in animals, ex-vivo studies were conducted on blood samples to examine and quantify the production of neutralizing antibodies capable of halting the infection process. These tests determined the strength of immunogenicity, indicating whether a significant antibody production occurred. Immunogenicity was evaluated in non-human primates using multiplex ELISA and cell culture neutralization assays.
- Verification of the efficacy of the VLA 2001 vaccine against SARS-CoV-2 in the cynomolgus macaque model:
Once immunogenicity was established, neutralization tests were performed to ascertain whether the antibodies produced could neutralize the virus, thereby preventing infection of cellular targets. In male cynomolgus macaques, two doses of the VLA2001 vaccine proved sufficient to elicit effective and protective immune responses. Following exposure to a high dose of SARS-CoV-2 virus, vaccinated groups demonstrated significant protection against viral replication in both the upper and lower respiratory tract, as well as reduced inflammation in lung tissue.
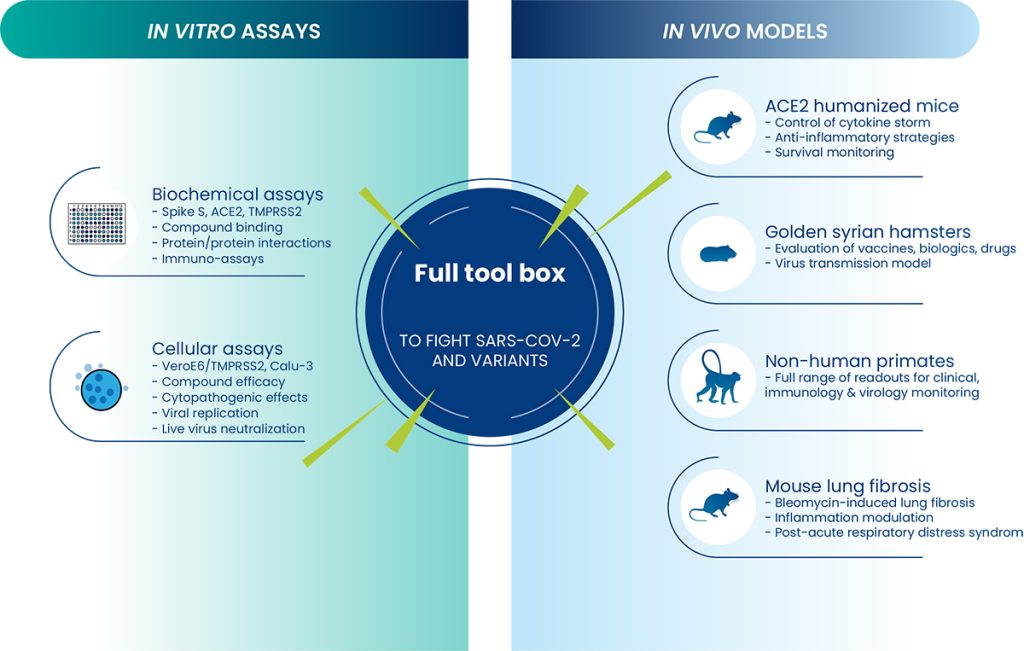
Oncodesign Services provides preclinical studies for the development of novel therapeutic solutions against infectious diseases, including vaccines
With BSL2 and BSL3 facilities and local partnerships, we manage infectious disease models in rodents and non-human primates. Additionally, our authorized in vitro study laboratories handle class 2 and 3 pathogens. Our services utilize an integrated array of biochemical and cellular tests, along with animal models infected with SARS-CoV-2 or other infectious agents. Furthermore, we employ modeling techniques to simulate pulmonary inflammatory complications.
Would you like to know more about our infectious models? Contact us!

![[PAPER]Immunogenicity and efficacy of VLA2001 vaccine against SARS-CoV-2 infection in male cynomolgus macaques](https://www.oncodesign-services.com/wp-content/uploads/2024/04/Paper-Highlight-10-1.png)
