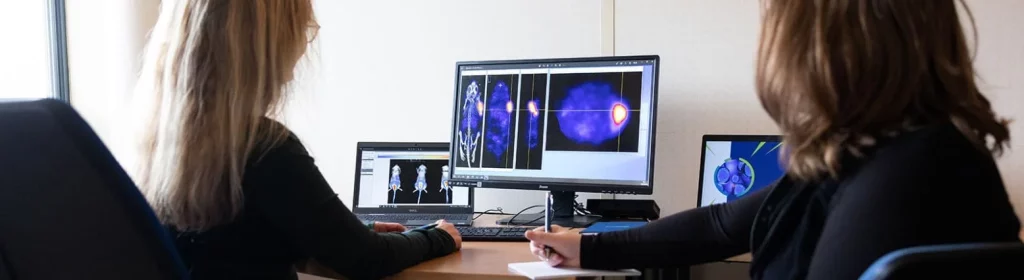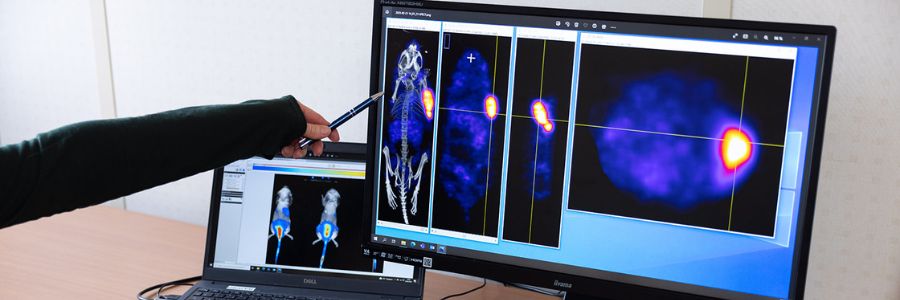


In the ever-evolving landscape of cancer treatments, precision has become paramount. Targeted molecules, honing in on specific receptors overexpressed in tumour cells, have reshaped therapeutic strategies. However, the next frontier extends beyond mere precision – it beckons a fusion of diagnosis and treatment. Enter theranostics, a ground-breaking approach that intertwines the power of targeted molecules with diagnostic tools.
This is the intriguing idea behind theranostic drugs. An existing targeted cancer drug is tagged with a radionuclide, which is then delivered directly to the tumour cells. The drug is often a biologic, such as an antibody, but in reality, any other form of targeted cancer drug could be used such as small molecules, peptides, nanoparticles and antibody fragments, as long as it is feasible to attach the radionuclide in some way. As well as killing cancer cells, there are radionuclides that are used as tracers in nuclear imaging such as SPECT (single photon emission computed tomography) and PET (positron emission tomography). This enables the cancer lesions to be visualised, tracked and monitored.
Iodine, the first theranostic
Although the idea of using targeted radionuclides as drugs is far from new – it dates back to the 1940s with the use of radioactive iodine to treat thyroid conditions – there has been a real boost to the field from the success of Lutathera®. But it’s not a straightforward path to market, and a full development package will be required for the regulators.
Lutathera® was approved in 2017 to treat somatostatin receptor positive gastro-entero-pancreatic neuroendocrine tumours (GEP-NETs). It combines the radionuclide 177Lu with the somatostatin receptor agonist tyrosine octotreotate, through the bifunctional chelator DOTA. Lutathera® was commercialised by Advanced Accelerator Applications, which was subsequently acquired by Novartis. The Swiss company has also bought another startup in the field, Endocyte, which developed another lutetium-labelled targeted radiopharmaceutical Pluvicto®, to treat prostate cancer. It has already been approved in a record time for the current clinical regulatory standards in this field.
The biotech sector has recognised the huge potential of theranostics, and various other potential products are currently working their way through the development pipeline.
Repurposing your cancer drug to the best theranostic candidate
Selecting an appropriate targeting drug is, of course, important. Ideally, it has affinity to overexpressed membrane receptors on tumour cells or to immunogenic markers produced in the microenvironment of the cancer lesion, reducing the likelihood of random uptake elsewhere in the body. A bifunctional chelator is added to the chosen biologic to ‘cage’ the radionuclide, this is to form a bond between the radionuclide and the drug. The stability between the radionuclide and the bifunctional chelator is critical since the energy released as the radionuclide decays can break the bond, enabling it to escape very easily.
Because the radionuclide is the therapeutic, the dose of the targeting biologic drug required is far lower than when used therapeutically on its own. It is not unusual for the dose to be 100, or even 1000, times lower. This means that any side-effects the drug itself has are unlikely to be an issue, as the dose is so small. Furthermore, much of the preclinical work, including toxicology studies, will already have been done for this part of the theranostic.However, development studies will certainly be required from the radiopharmaceutical point of view. The effect of the radionuclide, as it is attached to the drug, may change the properties of the biologic, including the pharmacokinetic and the biodistribution. It is also imperative to prove the efficacy of the treatment when the new therapeutic radiopharmaceutical is developed.
The effects of the radioactivity during circulation and elimination will also have to be assessed. A small molecule targeting drug is likely to be excreted via the urinary tract, with high uptake in the kidneys, and this may prove problematic. An antibody, meanwhile, is more likely to have protracted circulation in the bloodstream, and there may be effects on the bone marrow, or haematopoietic issues. So, it is important to perform dosimetry studies to assess the radiotoxicity in healthy organs based on how long it will circulate or remain in the body.
Logistics are another key consideration
The radionuclides will have relatively short half-lives; i.e for lutetium-177, it is 6.6 days. This means that radiopharmaceuticals cannot be manufactured and stored in bulk. The targeting part can, of course, be created in advance, but the radionuclide will need to be incorporated shortly before administration. Fortunately, cancer clinical sites will commonly have nuclear medicine lab capabilities on site where the radionuclide can be made available by a generator (Ga-68) or produced on site via a cyclotron (F-18). These can then be incorporated into the pre-made drug-plus-chelator complex within the lab, shortly before administration. For longer lived radionuclides (Lu-177) a centralised production in specialised CDMOs is preferred, where the production of the final radiopharmaceutical is also made upon request utilising established logistic solutions.
There are many variables that will need to be considered in the non-clinical regulatory package, as some are mentioned above. And, as an emerging field, the regulators are learning, too. But with a considerable number of products in development, and the effectiveness that has already been proven for a number of radiopharmaceuticals, the promise of the field is clear, as long as the development is done with care, and the manufacturing logistics are addressed at the outset.
Oncodesign Services has established a strategic alliance with Covalab, CheMatech and ABX-CRO the DRIVE-MRT to provide support and apply their expertise in the rationalization, design, and optimization of targeted radiopharmaceutical agents. We aim at designing and conducting comprehensive IND enabling preclinical packages in the field of Molecular RadioTherapy (MRT). Learn more about DRIVE-MRT.
About the author
This blog article was written by Dr Eftychia Koumarianou, head of Pharmaco-Imaging and Molecular RadioTherapy at Oncodesign Services. In her current role, Eftychia is involved in supporting pharmaceutical companies, who wish to enter the field of Theranostics.

A question ? A project around Molecular Radiotherapy ? Contact us !