Integrated laboratory solutions for your IND-enabling studies package
Oncodesign Services provides, with the support of dedicated partners, GLP studies to assess the safety of your drug.
Bioanalysis and GLP Analytical Services to support your drug development
Oncodesign Services offers various bioanalytical platforms for your regulatory needs in terms of sensitivity, specificity and throughput, at GLP (Good Laboratory Practice) and GcLP-grade.
Example of tailored bioanalytical services:
- Development and GLP-compliant validation of analytical methods for formulation
- GLP-compliant Dose formulation analysis
- Development and GLP-compliant validation of bioanalytical methods (LC-MS/MS, qPCR, dPCR, ECLIA, ELISA)
- GLP-compliant bioanalytical assays
- Immunogenicity risk assessment (IRA)
- In vivo toxicology

Our laboratories are certified Good Laboratory Practices (GLP) by the French regulatory authorities (ANSM). Our scientists develop, validate and perform your regulatory bioanalysis studies, in a quality environment and in accordance with Good Laboratory Practices.
Besides, our quality management system, under the responsibility of the Quality Assurance Manager, ensures the highest level of regulatory compliance along with the reliability and integrity of your experimental data. We carefully follow the guidelines for industry in the spirit of continuous quality improvement.
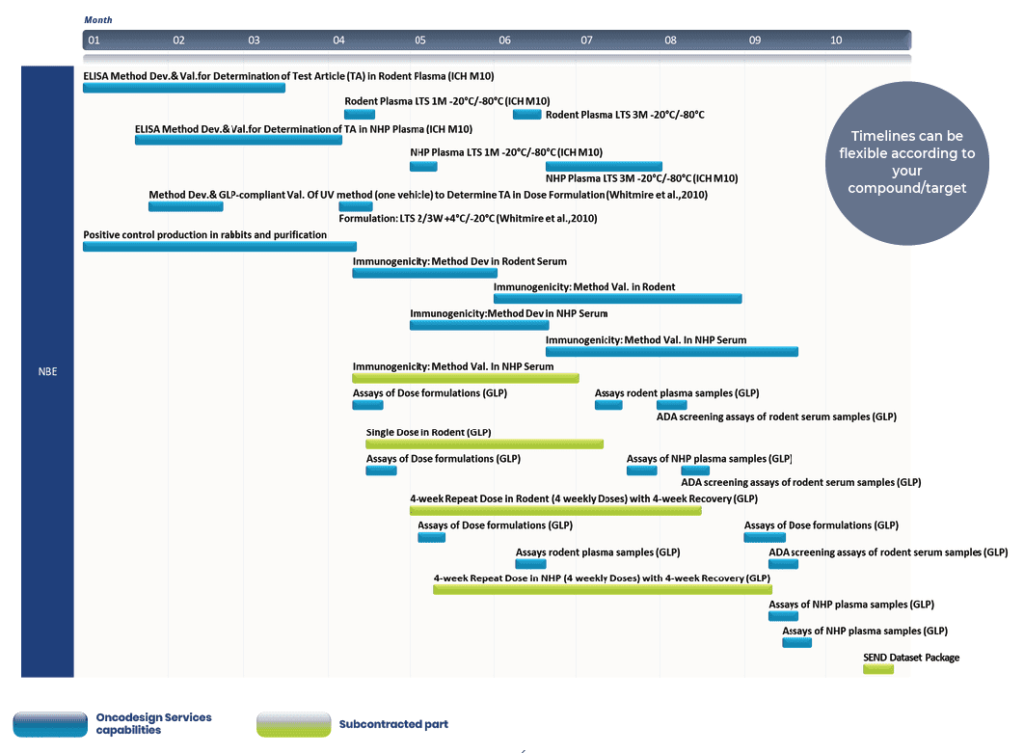
Typical example of generic IND enabling studies for new biological entities (NBE)
This generic example is based on regulatory applications being made in Europe or USA. All bioanalytical / formulation / immunogenicity / biomarkers were taken over by Oncodesign Services in our GLP test facility site at Paris Saclay (France). The other parts of the studies (mostly GLP in life phases) were subcontracted to dedicated partners.