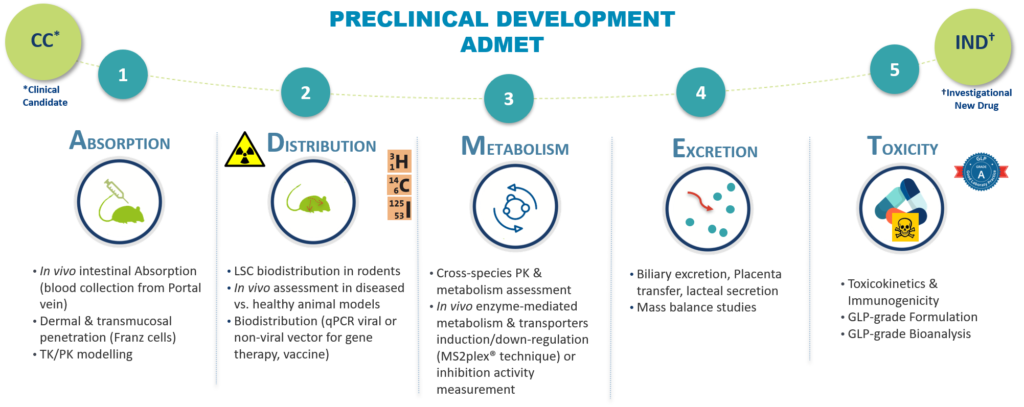
Oncodesign Services support your drug discovery project providing the pertinent ADME studies at the relevant time
The ADME studies are key in each preclinical stage of the drug discovery process, from high throughput screening (HTS), hit identification, lead optimization and finally the selection of a candidate molecule for clinical development.
An early characterization of these properties will ensure selection of compounds with acceptable pharmacokinetic (ADME) characteristics to guarantee efficacy while limiting adverse effects and optimizing development time.
Oncodesign services help you in your drug discovery project providing the pertinent ADME studies at the relevant time.
What is ADME testing?
ADME testing is the abreviation of “Absorption, Distribution, Metabolism and Excretion”. It is a set of experiments conducted in drug development to assess how substance is absorbed, distributed, metabolized and eliminated by the body.
- Absorption
How is the drug absorbed into the body, how fast?
- Distribution
Where does the drug go? Stay mainly in the plasma, binding to plasmatic proteins, binding to tissues?
- Metabolism/Transport
How long does the drug stay in its unchanged form in the body? which metabolites are generated and in what proportion?
Are there any risks of drug-drug interactions (DDI)?
Are transporters involved in the ADME process: drug-drug interaction DDI, nonlinear PK, lack of correlation between species…?
- Excretion
Where is the drug excreted: urine, feces, under which form (changed/ unchanged)?
ADME assays provide valuable and complete data of safety and risk about the compound through preclinical drug development. Indeed, an IND submission requires an assessment of the pharmacokinetics and information on the ADME properties.
ADME assays are recommended at each stage of drug discovery.
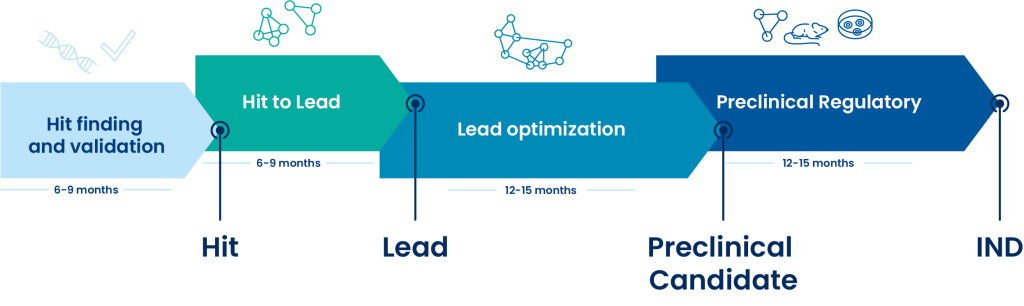
Oncodesign Services, a leading CRO for ADME studies
The Oncodesign Services ADME department gather experts in Drug Discovery to support your research programs. Our laboratories are BSL1, BSL2 and BSL3 and AAALAC accredited for our animal care and use program.
Our ADME services include:
- Physicochemical properties
- In vitro ADME testing
- Intestinal/ skin Permeability
- Drug-drug interactions
- In vitro/ in vivo metabolism
- Toxicity studies (ADME-Tox)
Integrated services to the screening cascades
At Oncodesign Services, we customize screening cascade adapted to discovery stage and project. The ADME assays are routinely monitored by a range of reference compounds. Turnaround times from compound reception to data reporting are between 5 to 25 working days depending on selected assays
Assays are scalable towards higher throughput screening (HTS) by using robotic platform and multiple plate format.
Based on our extensive experience, Oncodesign Services’ team offers support and all along your preclinical projects with fast cycles times and an access to latest innovative technologies. The assays list below is not exhaustive and can be customized according to your needs.
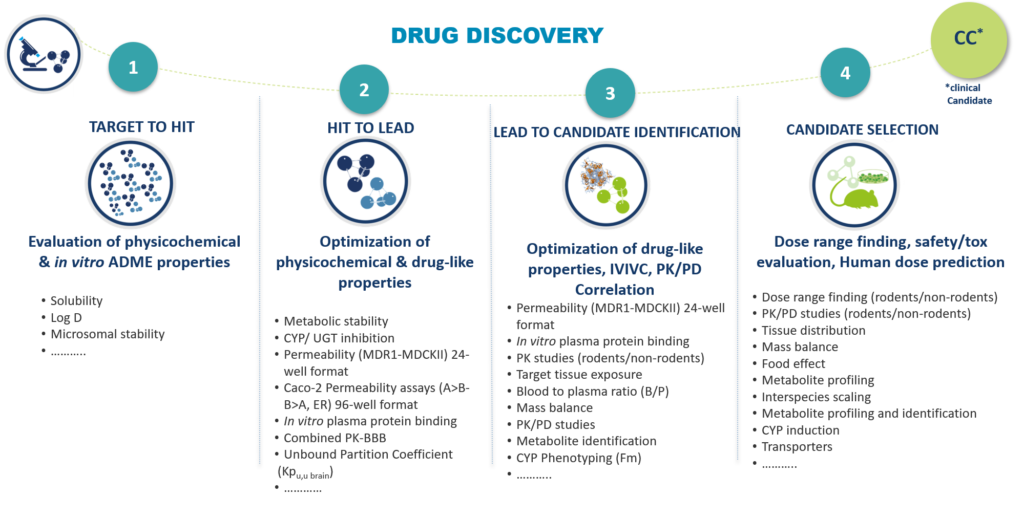
The DMPK/Bioanalyses unit of Oncodesign Services works closely with the departments responsible for medicinal chemistry, in vitro screening, in vivo proof of concept, to generate high quality hits, leads and drug candidates in a timely manner very short.
Once the candidate has been selected, Oncodesign Services can continue to help you build your IND package for submission to authorities.
We offer you our expertise in DMPK & non-GLP/GLP bioanalysis, to support you in the complete characterization of the ADME properties and the evaluation of the toxicity of your future clinicate candidate.