


Anti-Drug Antibody (ADA) Assay services to support your Drug Discovery programs
ADA (Anti-Drug Antibody) assays conducted throughout the drug development process are essential for immunogenicity risk assessment to aid in selecting a suitable drug candidate.
Oncodesign Services is your partner in drug discovery and preclinical studies. Our expertise in pharmacology, including anti-drug antibody (ADA) assays, ensures that your drug development process is efficient, safe, and effective.
Overview of anti-drug antibody (ADA) assay
ADAs are immune responses against therapeutic drugs, potentially impacting their efficacy and safety. ADA assays allow to detect and characterize these antibodies, ensuring drug candidates do not trigger harmful immune reactions.
ADA assays are commonly based on a tier approach:
- Screening Assays: Usually ELISA (enzyme-linked immunosorbent assays) or ECL (electrochemiluminescence) assays, these are designed to detect the presence of anti-drug antibodies.
- Confirmatory Assays: These are used to confirm the specificity of the antibodies detected in the screening assay, often using a competitive format where the drug is used to inhibit antibody binding.
- Characterization assays (e.g. Titration assay, Nab assay, ADA isotyping…)
Why use anti-drug antibody (ADA) assay early in drug discovery & preclinical research?
Early implementation of ADA testing is essential for assessing drug safety and efficacy, identifying immunogenicity risks, and optimizing drug candidates. Using in vitro assays, our preclinical studies efficiently detect ADAs and provide critical data for regulatory submissions and subsequent development stages.
Our immunogenicity platform offers solutions and services in ADA assessment involving these typical readout:
-
Production, purification and characterization of ADA positive control
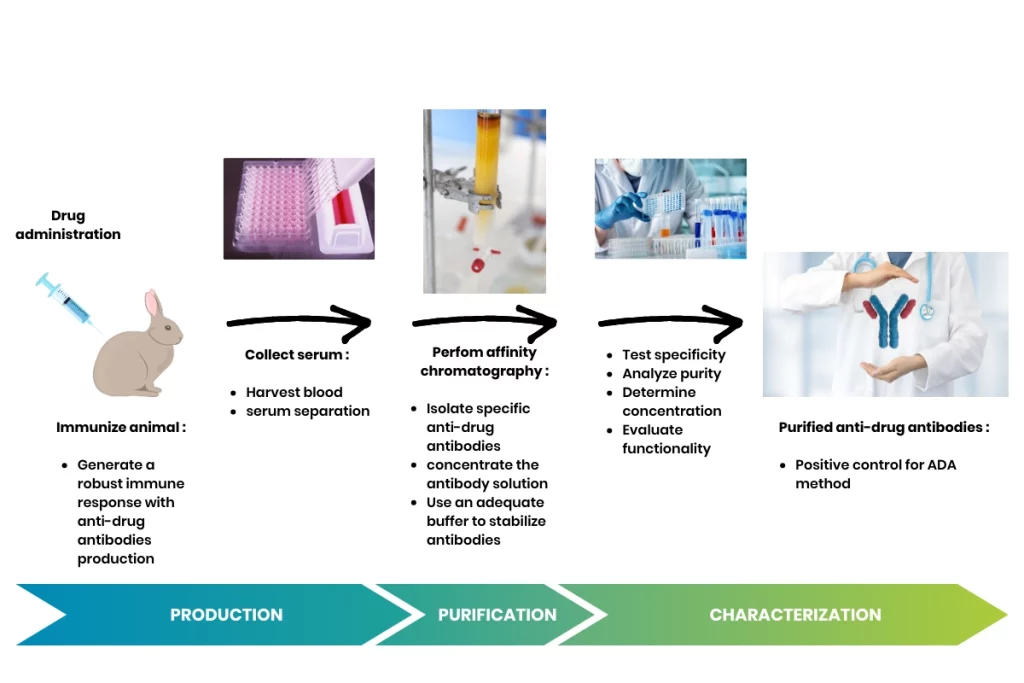
Detailed protocol of production, purification and characterization of ADA positive control
-
ADA method development (bridging ECLA on Mesoscale Discovery® technology, or ELISA)

- Screening assay / Confirmation assay
- Characterization assays:
- Titration assay
- ADA isotyping
- NAb assay (Cell-based assay (CBA), competitive ligand binding assay (CLBA))
- Preparation of specific reagents: Labeled drug (Biotin, ECL-Sulfo-Tag, enzyme, carrier)
- Determination of optimal experimental conditions (incubations, buffers…)
- Sample pre-treatment step: dissociation of complexes “drug/ADA” and/or drug extraction to improve drug tolerance of the method
-
ADA method validation
ADA method will be qualified or validated following current international guidelines (FDA1, EMA2) and white papers from industry (EBF3, EIP4, AAPS5).
Critical parameters to be determined:
- Minimum Required Dilution (MRD)
- Sensitivity
- Drug tolerance
- Screening cutpoints (SCP)
- Determination of QC levels
- Precision of the method
Other parameters to be determined (according to immunogenicity risk assessement of the drug, and of the stage of the drug development (preclinic, clinic)):
- Confirmatory cutpoints (CCP) / Titration cutpoint (TCP)
- Specificity
- Selectivity
- Stability
- Robustness
-
ADA sample assay from preclinical studies (GLP) and clinical trials (GcLP)
Learn more about how ADA sample assay are used: Detection of anti-drug “X” antibodies in specimens from toxicity study in cynomolgus monkey including recovery and toxicokinetics
| With 30 years of experience, Oncodesign Services, a leading CRO in preclinical research, offers GLP-grade ADA assays using advanced detection methods in preclinical (GLP) and clinical (GcLP) samples. We provide comprehensive in vitro ADA testing to detect ADAs, deliver regulatory data, and assess drug safety and immunogenicity risks, supporting faster decision-making in drug development. Contact us to learn how our integrated drug discovery platform can assist your research. |  |
-
References
1.FDA
- Food and Drug Administration: Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for Industry: Immunogenicity Testing of Therapeutic Protein Products —Developing and Validating Assays for Anti-Drug Antibody Detection (January 2019).
- EMA
- 18 May 2017 EMEA/CHMP/BMWP/14327/2006 Rev 1, Guideline on Immunogenicity assessment of therapeutic proteins.
- EBF
- Laurén A. et al. A strategic approach to nonclinical immunogenicity assessment: a recommendation from the European Bioanalysis Forum. White Paper – Bioanalysis (2021)
- Kramer D. Risk Based Approaches to Immunogenicity – EBF Training Day: Practical Aspects of Immunogenicity (2021)
- Dreher I. Towards an EBF Recommendation on NAb – Training Day: Managing the Practical Aspects of Immunogenicity (2021)
- EIP
- Devanarayan V. Screening & Confirmatory Cut-Points – Brief Overview & Answers to some FAQs. EIP Symposium, Immunogenicity of Biopharmaceuticals (2009)
- Devanarayan V. Practical advice & insights on Immunogenicity cut points and some assay validation parameters. EIP Symposium (2019)
- Kramer D. ADA Testing in Repeated Dose Toxicity Studies – Strategies EIP Symposium (2020)
- Devanarayan V. Practical Advice on When and How to Perform In-Study Cut-points. EIP Training Couse, April 24, 2023
- AAPS
- Shankar G. et al. Assessment and Reporting of the Clinical Immunogenicity of Therapeutic Proteins and Peptides—Harmonized Terminology and Tactical Recommendations. AAPS J. (2014)
- Devanarayan V. et al. Recommendations for Systematic Statistical Computation of Immunogenicity Cut Points. AAPS J. (2017)
- Myler H. et al. Meeting Report – Report on the AAPS Immunogenicity Guidance Forum. The AAPS Journal (2019)
- Civoli F. et al. Recommendations for the Development and Validation of Immunogenicity Assays in Support of Biosimilar Programs. AAPS J. (202
A request about our ADA assay ? Contact-us !



