

Pharmaco-imaging, a non-invasive approach to support proof of concept in pharmacology
Imaging is a non-invasive tool for pharmacology enabling to significantly reduce the number of animals and to efficiently monitor:
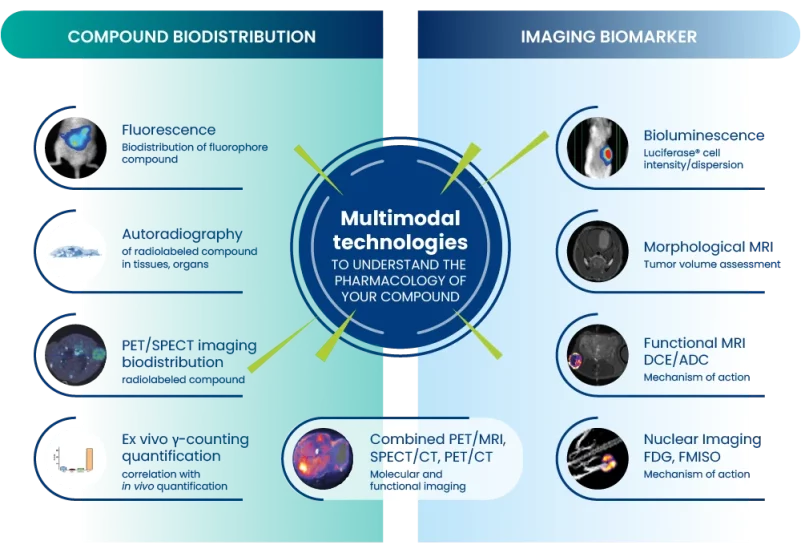
Oncodesign Services has added pharmaco-imaging to its pharmacology platform in 2004, integrating along the years imaging multimodalities:
- MRI 4.7T
- Nuclear imaging (PET/CT, SPECT/CT, PET/MR)
- Optical imaging (Bioluminescence, Fluorescence)
- Ultrasound
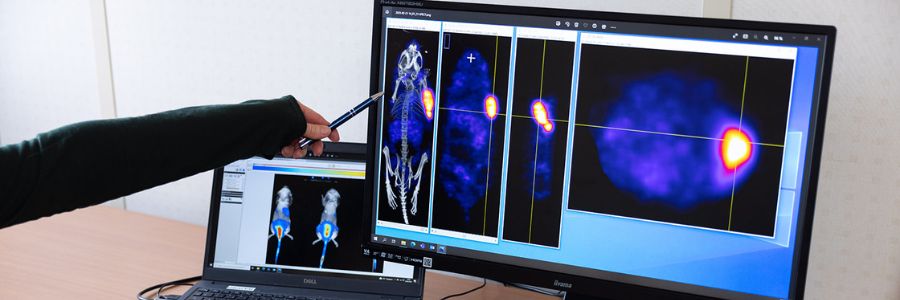
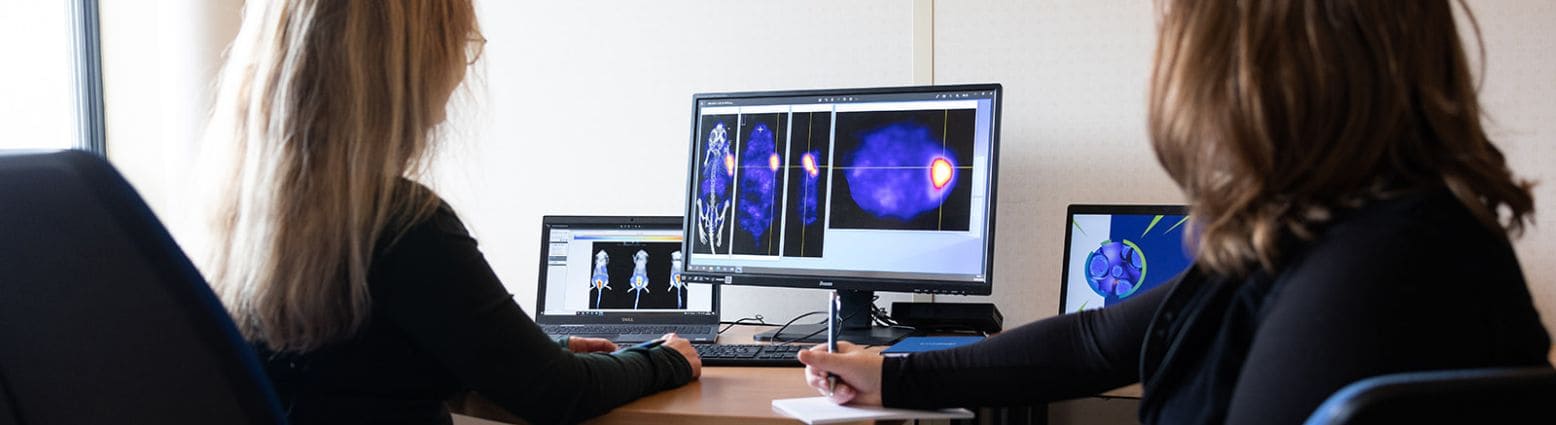
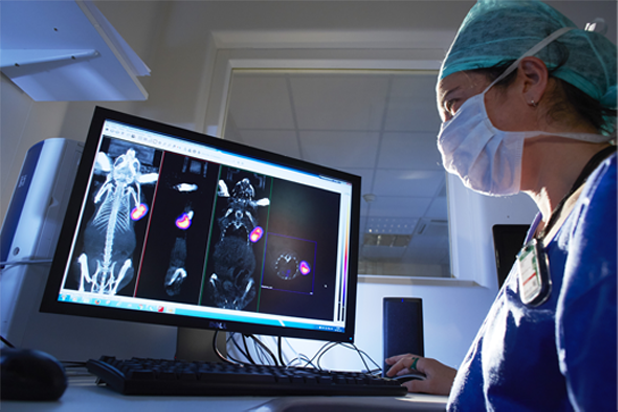
Oncodesign Services’ in vivo multi-modal imaging platform can be used for biodistribution studies, tumor tracking and to detect biomarkers of activity. Pharmaco-imaging studies often utilize radioisotopes, leveraging the capabilities of our bioconjugation and radiochemistry expertise. It is applicable to small molecules, biologics, nanoparticles.
Oncodesign Services support radiopharmaceuticals development for Theranostics.
DRIVE-MRT: a premium “nuclear medicine” solution in Oncology
DRIVE-MRT is part of our continuous improvement in the integrated drug discovery support. Oncodesign Services and its strategic partners Covalab, CheMatech, and ABX-CRO are applying their expertise in the rationalization, design, and optimization of targeted radiopharmaceutical agents.

Pharmimage® – a non-invasive pharmaco-imaging capability
Our technology module, Pharmimage® is designed to monitor the effect of treatments and to define effective translational biomarkers in precision medicine. It helps to answer questions using non–invasive, whole–body techniques:
- Is the target expressed? (Diagnosis of the disease, heterogeneity of target expression, distribution of the target in metastatic cancer, CNS, cardiology, etc.)
- Does the drug reach the target? (Pharmacokinetics, biodistribution, blood–brain barrier crossing, etc.)
- Is the target inhibited/activated, and does this induce biochemical and biological changes? (Pharmacodynamics, immunomonitoring, optimal biological dose, etc.)
- Are there any potential toxicities and drug interactions? (Safety, study on drug combinations, metabolism, etc.)
- Are these changes related to a clinical criterion? (Efficacy of a new treatment, monitoring of the immune response to a vaccine, emergence of resistance in precision medicine, etc.)
- Can subpopulations of responders be determined? (Translational biomarker)