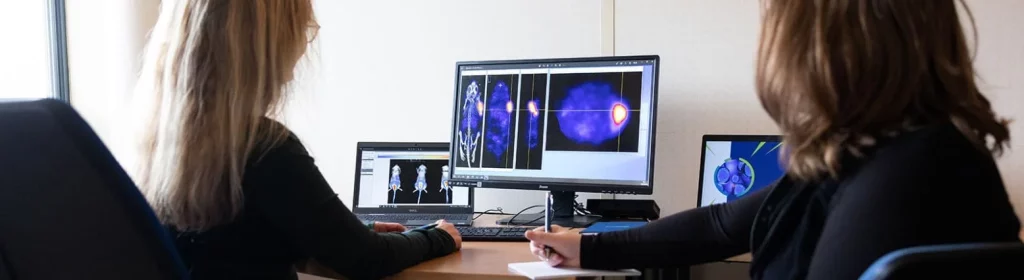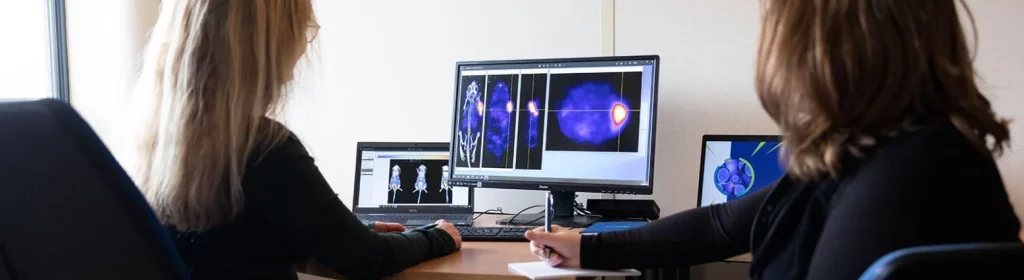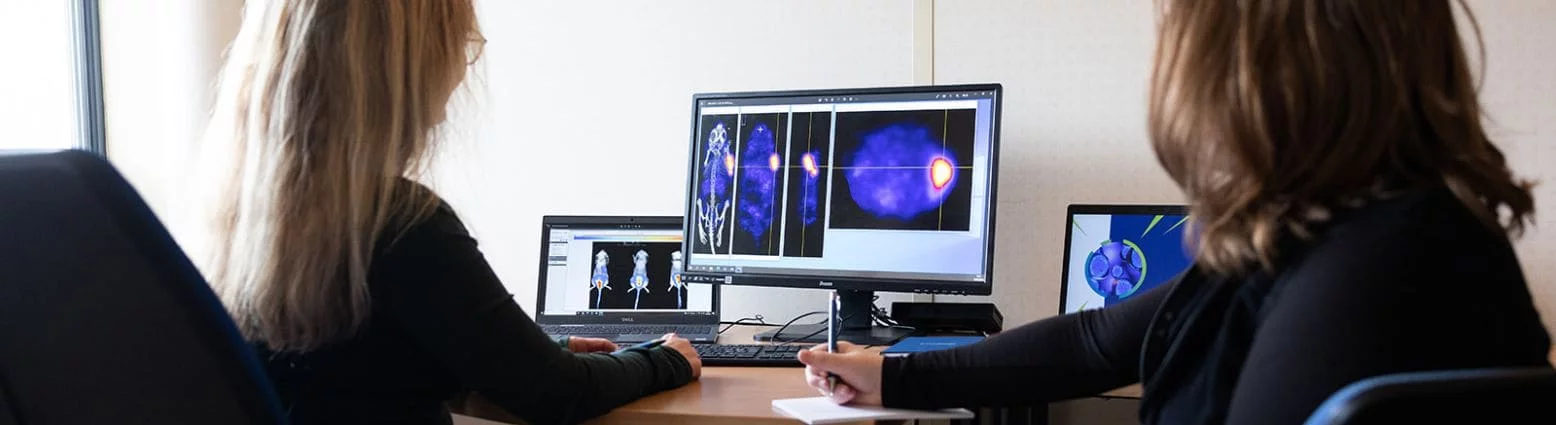


A premium “nuclear medicine” solution in Oncology
Oncodesign Services and its preferred specialized partners Covalab, CheMatech, and ABX-CRO are applying their expertise in the rationalization, design, and optimization of targeted radiopharmaceutical agents.
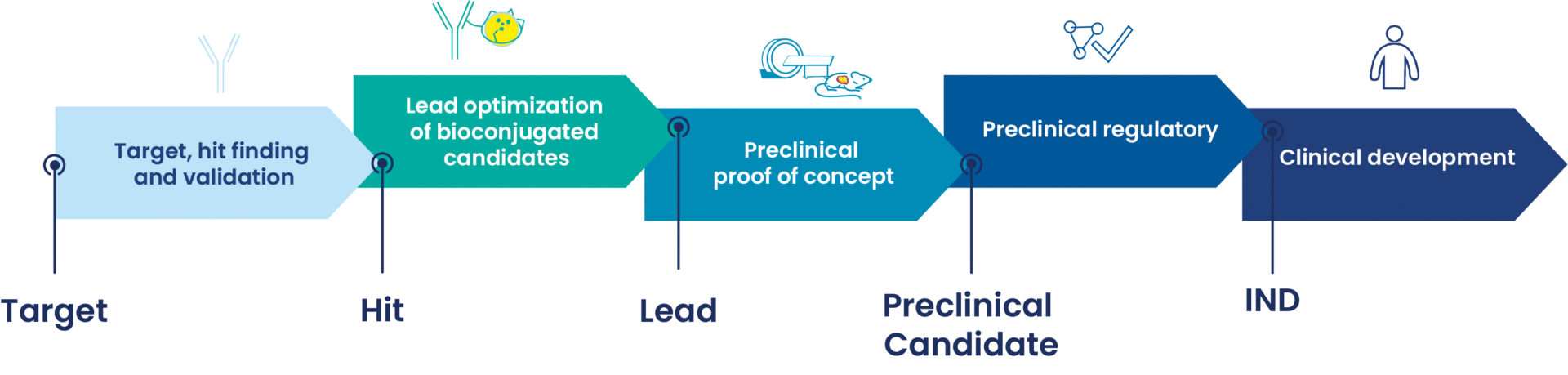
DRIVE-MRT encompasses all the skills, experience and technological platforms needed to generate new targeted radiopharmaceutical products in oncology.
We enable faster and cost optimized development of your therapeutic, diagnostic or theranostic entity from bioconjugation and radiolabeling up to IND-enabling studies, even first-in-human.
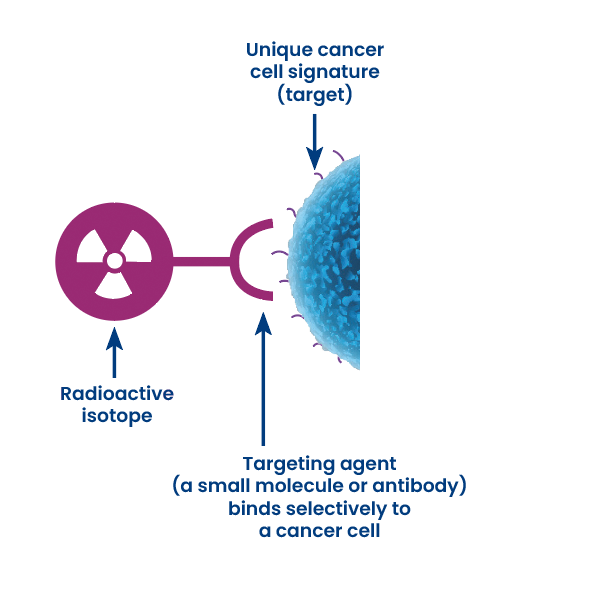
Radiopharmaceutical development requires specific and dedicated expertise whatever the step you are at!
-
Hit finding and validation
What is the most specific vector for a Targeted RadioTherapy between full antibody and small fragments?
What is the best strategy to identify and select these various biological vectors?
We guide you in selecting the best high affinity antibody or fragments to be used for labeling & in vivo imaging
-
Lead optimization of bioconjugated candidates
Do you have a process to bioconjugate and radiolabel your compound?
When linked with a chelating agent, do the compound have the same affinity, target engagement, in vivo biodistribution?
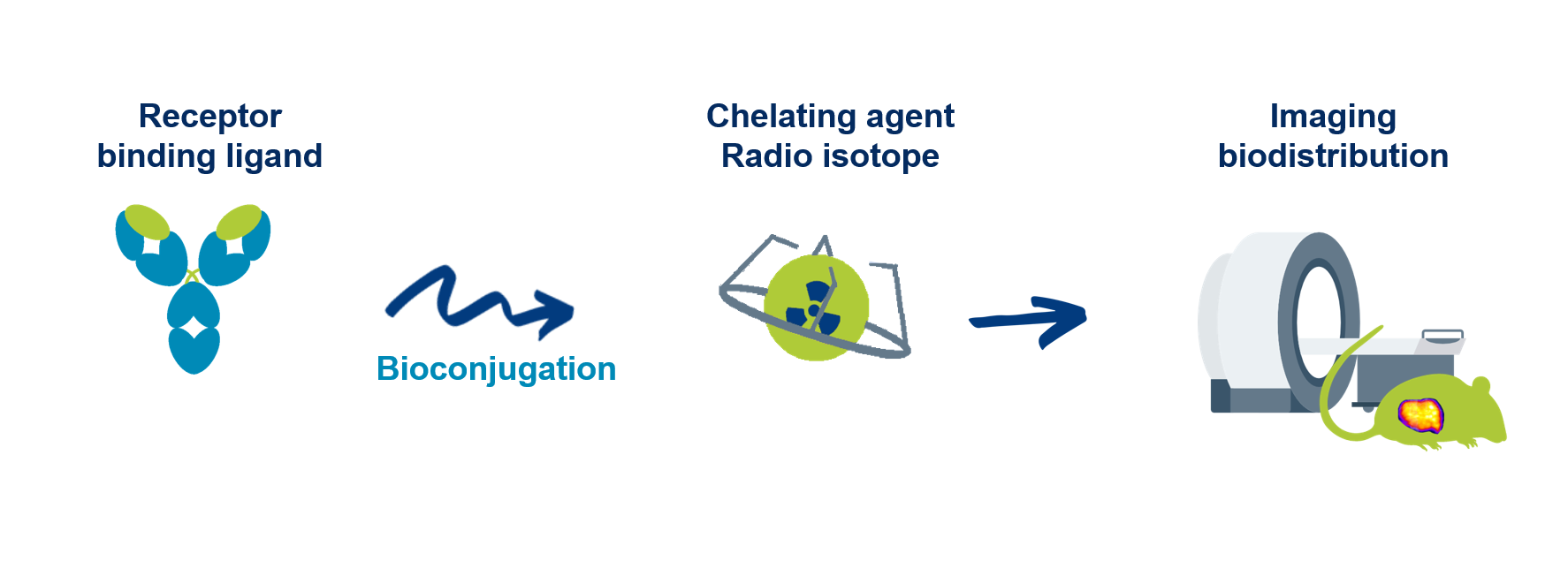
The key objective of the Lead optimization phase is to define the best strategy of bioconjugation, selection of linker (PEG, BSA binder…), and radiolabeling in order to select an optimal bioconjugated compound (and possibly 2-3 back-up compounds) with improved binding demonstrating a robust selectivity and in vivo biodistribution.
-
Preclinical proof of concept
To undergo regulatory IND-enabling studies, critical questions require to be addressed. What is the proof of concept and the pharmaceutical developability behind new target or biomarker? What is the therapeutic preclinical efficacy of this new molecule?
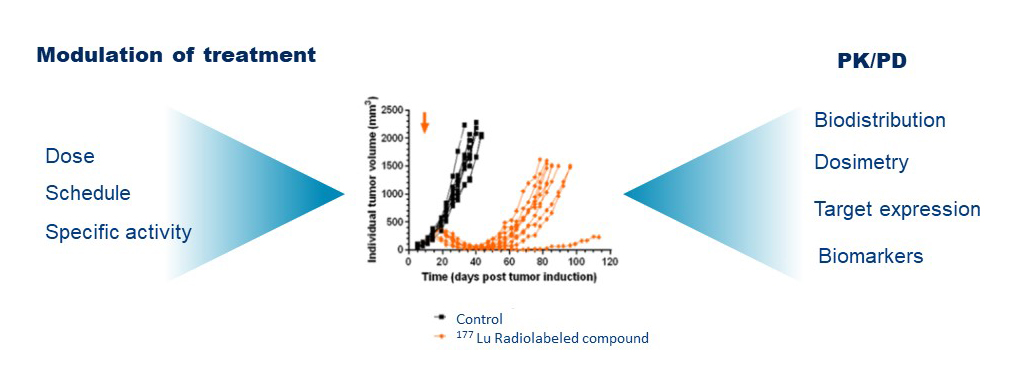
We design and implement preclinical studies to demonstrate the translational validity of novel pharmaceuticals as imaging or therapeutic agents using in vitro, ex vivo and in vivo assays in the appropriate models.
-
Preclinical Regulatory
How to define the regulatory preclinical package for a radiopharmaceutical for diagnostic or therapeutic application?
We can provide you with a cGMP service to produce starting materials and intermediates. CheMatech conducts in-house cGMP manufacturing of chelating agents from grams to kilo. ABX-CRO will support your Radiopharmaceutical Development and dosimetry.
-
Clinical Development
What are the essentials for a successful preclinical strategy and applications for conducting radiopharmaceutical clinical trials?
- Close relationship between preclinical and clinical project managers to ensure continuity of your project
- Integrated protocol development campaigns with joint Key Opinion Leader panels from target (e.g. oncology,) and technical (nuclear medicine, radiology) medical fields
- Extensive project de-risking using advanced literature-based sample size estimations, and risk factor simulations
- Pharmacovigilance: Patient safety is critical and we understand the specific safety requirements associated with the use of radionuclides and imaging technologies.
Our expertise
Hit Finding and validation
- DNA / protein /hapten immunization
- Antibody Format selection (Fab, ScFv, VHH) from immune or naive library
- Antibody selection and validation
- Small scale production, purification and validation
- Site specific bioconjugation (immunoconjugates)

Lead optimization of bioconjugated candidates
- In vitro compound screening
- Bioconjugation strategy
- Optimization of chelating agent
- Optimization of linker
- In vitro Biochemistry & Biology
- In vitro and in vivo DMPK
- Radiolabeling
- Imaging biodistribution

Preclinical proof of concept
- In vitro and in vivo DMPK
- In vivo Pharmacology
- Efficacy POC
- Pharmaco-imaging
- PK & Time Activity Curves
- Combination studies with SOC
- Early Scale-up and formulation
- Early Safety assessment

Preclinical Regulatory
- Project Management and regulatory filling
- Radiopharmaceutical Development
- Dosimetry (QDOSE® software)
- Regulatory Toxicity studies

Clinical Development
- Clinical project management
- Dosimetry (QDOSE® software)
- Data management & biostatistics
- Medical Writing
- Medical Monitoring
- Pharmacovigilance
Thanks to our collective experience, we can offer you a package of this premium integrated solution to avoid problems!