

CRO services to support your radiopharmaceuticals drug development program
Radiopharmaceuticals are drugs containing radioactive isotopes. They are used for diagnostic or therapeutic agents in nuclear medicine.
The development of radiopharmaceuticals inquires several stages including discovery (to identify and optimize a target that may be treated using radiopharmaceuticals) and preclinical studies (to evaluate the safety, pharmacokinetics and efficacy of the radiopharmaceutical candidate).
Oncodesign Services, a leading CRO specialized in Drug Discovery and preclinical services, support you in your radiopharmaceuticals drug development program.
Radiopharmaceutical development requires specific and dedicated expertise
From synthesis of bioconjugated compounds to IND for radiopharmaceutical compounds, Oncodesign Services offers stand-alone (SOLO) or integrated development package (DRIVE-MRT) for radiopharmaceuticals.
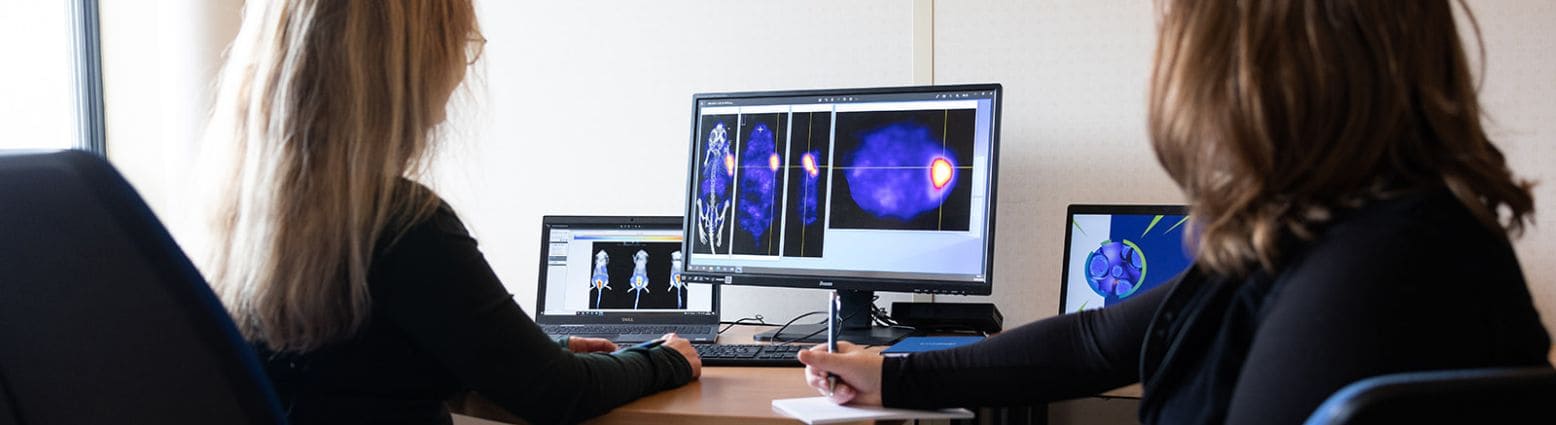
Oncodesign Services has a unique offering dedicated to pre-clinical evaluation of radiopharmaceuticals, with a combination of our skills in radiochemistry and in vivo pharmaco-imaging in order to assess systemic radiotherapy efficacy.
Our services include:
Bioconjugaison & radiolabeling
Binding assessement biological samples
Biodistribution
Efficacy POC
Dosimetry analysis
Toxicologie studies
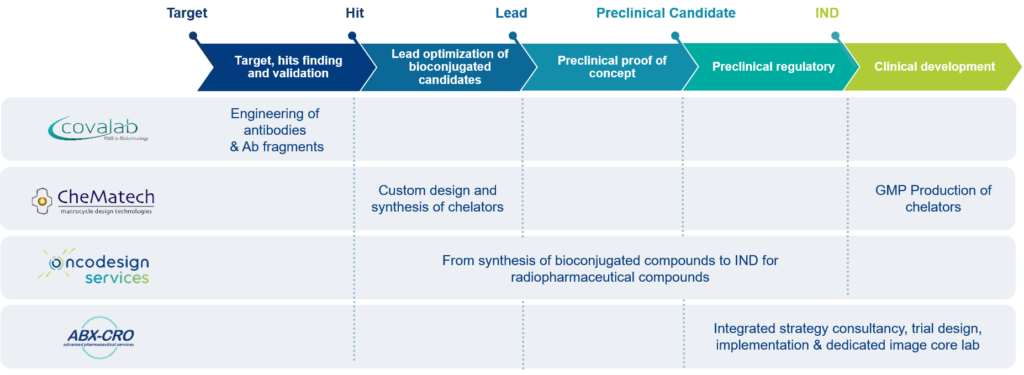
Drive-MRT : A premium “nuclear medicine” solution in Oncology
With Drive-MRT, Oncodesign Services and its strategic partners Covalab, CheMatech, and ABX-CRO are applying their expertise in the rationalization, design, and optimization of targeted radiopharmaceutical agents. This global network of experts can help you to manage efficiently your radiopharmaceuticals drug development and take the right decision at the right time.