


Oncodesign Services has ABSL2 and ABSL3 facilities and local partnerships allowing infectious disease models to be run in rodent and NHP. In addition, we have in vitro laboratories certified to handle BSL2 and BSL3 pathogens.
Oncodesign Services can offer integrated drug discovery and preclinical programs (INPACT) for antivirals, from chemistry, screening to in vivo proof of concept. With all the capabilities under the same roof, we can offer fast cycle times.
COVID-19
Oncodesign Services offers a complete portfolio of in vivo & in vitro solutions to evaluate products against SARS-CoV-2 infection. This offer was swiftly deployed in 2020, allowing – with rigor and safety – the implementation of specific in vitro and in vivo pharmacology studies to evaluate new prophylactic, therapeutic or vaccinal approaches to fight COVID-19.
Other pathogens
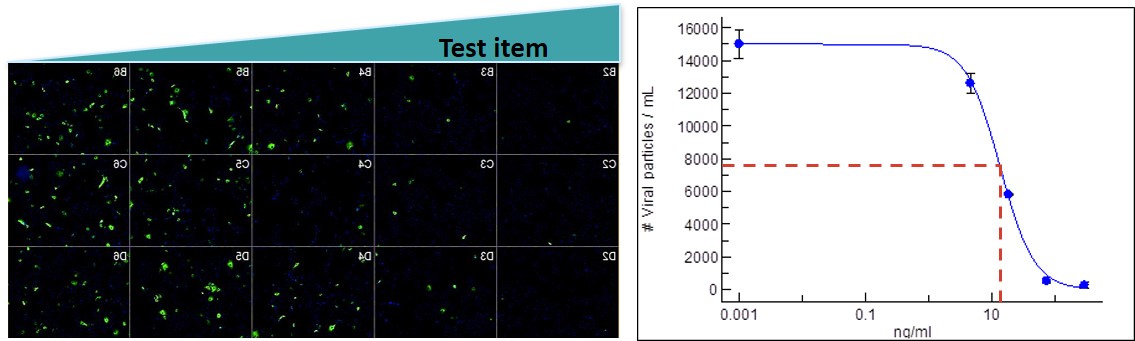
In vitro biology
Oncodesign Services provides typical assays offered for viral studies: viral expression by cell imaging, viral-induced cytotoxicity, antibody neutralization, compound binding, inhibition of infectivity and cytokine expression.
Depending on your project, we can acquire new pathogens. Oncodesign Services is already certified to handle a number of pathogens in vitro:
- Herpes (HSV-1 and -2)
- Arboviruses (Chikungunya, Ross river, Dengue, Semliki, Mayaro, O’nyong nyong, Zika)
- Human immunodeficiency virus (HIV-1 and -2)
- Influenza A/H1N1
- SARS-CoV-2 with different variants (D614G, alpha, beta, gamma, delta, omicron, BA.5, JN.1, HK.3)
- Human rhinovirus and parainfluenza 3
Targeted cells for each pathogen are available and validated. Classical protocol of infection is followed. Readouts include cell viability (cytotoxicity of candidate without virus), colorimetric assay when cytopathogen effects exhibited, viral replication with commercial kit when available, viral load by RT-qPCR (relative value) and digital PCR (absolute value in number of viral copy), possibility of immufluorescence with HCS imaging (ImageXpress® Pico technology).
In vivo models
We also propose the TC-1 syngeneic (C57BL/6) mouse tumor model (HPV+) for the evaluation of HPV-targeting therapies. In this model, the recipient mice mount a HPV specific CD8+ T cell response that can monitored by flow cytometry (Dextramer HPV-E7 staining).
Other models with our partners
Through the Infectious Disease Model and Innovative Therapies (IDMIT center, CEA, Fontenay, Paris, France) as a partner, we can offer NHP studies in other pathogens.
The IDMIT Center has experience running the following pathogenic models in NHP:
- HIV/SIV
- SARS-CoV-2
- Monkey Pox
- Tuberculosis
- Pertussis (baboons)
- Yellow Fever
- Zika
- Dengue
- Chikungunya
- Chlamydia
- Flu (+/- Strepto. Pneumoniae superinfection)
- Malaria
- Trypanosome (Chagas)
- CBRN
Please ask us about your pathogen of interest.
image: validation of antibody neutralization capacity
A question ? A request for your project ? Contact us !
