


Help the development of vaccine and innovative therapies against arboviruses
Arboviruses (short for “arthropod-borne viruses”), including dengue, yellow fever, Zika, and Chikungunya, are significant human pathogens transmitted by mosquitoes. They pose an increasing public health challenge, in particular in tropical and subtropical regions.
At Oncodesign Services, we are engaged and committed to help finding therapeutic solutions and innovative treatments against on human infectious diseases, including arboviruses, by providing access to facilities and cutting edge equipment for R&D programs.
Preclinical evaluation for arbovirus vaccine and new therapies candidate
For preclinical evaluations of new arbovirus vaccine candidates, representative in vitro and in vivo experimental systems are essential to assess specific immune responses. These models help researchers develop vaccines, therapeutics, and other control measures.
At Oncodesign Services, our in vitro laboratories are certified to handle BSL2 and BSL3 pathogens related to arboviruses, such as :
- Chikungunya models
- Zika models
- Dengue models
- Ross river models
- Semliki models
- Mayaro models
- O’nyong nyong models
We provide typical assays for viral studies on arboviruses:
- colorimetric assay when cytopathogen effects exhibited
- viral load by RT-qPCR (relative value) and digital PCR (absolute value in number of viral copy)
- viral-induced cytotoxicity
- cell viability
- viral expression by cell imaging
- viral replication with commercial kit
- antibody neutralization
- compound binding
- inhibition of infectivity and cytokine expression
- immufluorescence with HCS imaging (ImageXpress® Pico technology)
With our network of partners, we can also provide in vivo testing until clinical trials to study the efficacy of new therapies against Arboviruses.
Oncodesign Services can offer integrated drug discovery and preclinical programs (INPACT) for antivirals, from chemistry, screening to in vivo proof of concept. With all the capabilities under the same roof, we can offer fast cycle times.
Case study :
-
Chikungunya
- Capacity of antibody (Ab) candidate to neutralize panel of CHIKV (LR2006,Carribean , SH287, 37997 and Zs Green)
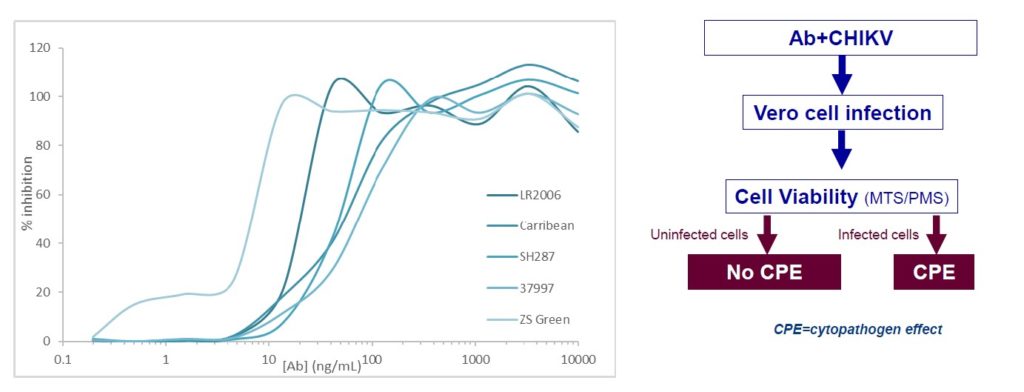
- Evaluation of CHIKV LR2006 resistance
under antibodies (Ab#1,2,3,4) pressure along rounds
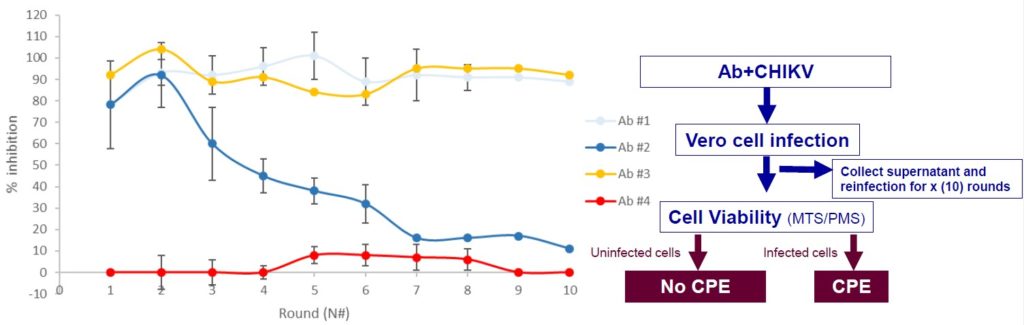
Different profiles can be obtained depending on the antibody
evaluated. Ab#1 and Ab#3 are selected- Antibody-mediated enhancement (ADE) of CHIKV infection in mouse macrophages
Mouse macrophages (Raw 264 cell model)
Viral replication is measured by quantifying CPE
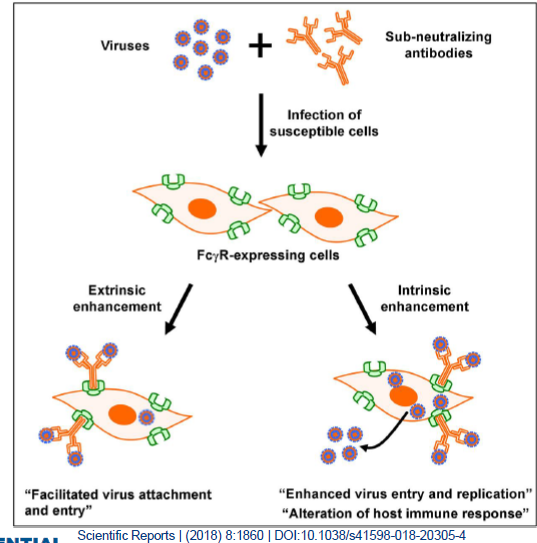
-
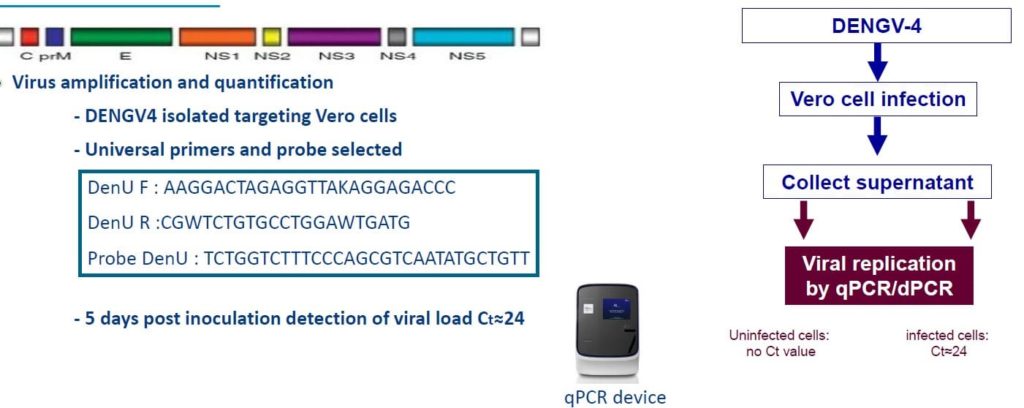
Dengue
- DENGV4 mRNA quantification
In vitro application to screen antiviral candidates
Other pathogens available at Oncodesign Services in vitro laboratories:
- SARS-CoV-2 with different variants (D614G, alpha, beta, gamma, delta, omicron, BA.5, JN.1, HK.3)
- Influenza A/H1N1
- Human rhinovirus and parainfluenza3
- Herpes (HSV-1 and -2)
- Human immunodeficiency virus (HIV-1 and -2)

