


Preclinical CRO Services for Inflammatory Diseases of the Skin
Dermatological diseases or symptoms, including but not restricted to skin inflammation , affect patients’ lives, not only from a medical, but also from a social point of view. Rodent models of skin inflammation are helpful to decipher the mechanisms of disease triggering or sustaining in patients.
Oncodesign Services offers access to several standard preclinical models addressing a variety of skin pathologies, and provides CRO support for de novo development of new skin inflammation models recently described in literature.
Typical readouts for skin inflammation
In vivo readouts :
- Clinical scoring
- Body weight
- Real-time scratching (itch)
Ex vivo readouts :
- Fibrosis score
- Histopathology
- Biomarker/drug monitoring
- Gene expression in skin, by qPCR/dPCR
- Trans-epithelial water loss (TEWL)
Discover Oncodesign Services offers for skin inflammation diseases
-
Ex Vivo assays
Primary human skin explants from healthy donors are used in a Franz cell device and treated either in the top chamber to simulate topical application or in the bottom chamber to simulate systemic exposure.
Typically used to analyze:
- Target engagement
- Skin barrier functionality
- Biomarker expression or inflammatory response, typically by PCR.
-
In vitro capabilities
- Human primary keratinocytes
- Assays: IL-8 secretion
- Human reconstituted epidermidis (3D)
- Assays: Stimulation of inflammatory pathways
- Co-culture with bacterial supernatant │
- Differentiation │ Proliferation │Triglyceride synthesis
- Human sebocytes
- Assays: Triglyceride synthesis
- Human primary keratinocytes
-
In Vivo models
Oncodesign Services has developed several robust models of skin diseases available as CRO services to support your R&D programs.
This is a non-exhaustive list, get in touch if you cannot find your desired model in the list.
- Psoriasis model in mice, induced by Imiquimod (1)
- Psoriasis model in rats, induced by Imiquimod (2)
- Skin scleroderma/fibrosis model in mice, induced by Bleomycin, Topoisomerase-I peptide-loaded dendritic cells (3), Topoisomerase-I and CFA (4)
- Atopic dermatitis model in mice, induced by Calcipotriol (5), DNFB (6) or HDM (7)
- Pruritogen itch model in mice, induced by Chloroquine, Substance P or Imiquimod (8)
- Acnea model in rats and mice, induced by Sebaceous gland atrophy (9)
Exemple : ex vivo human skin biopsy
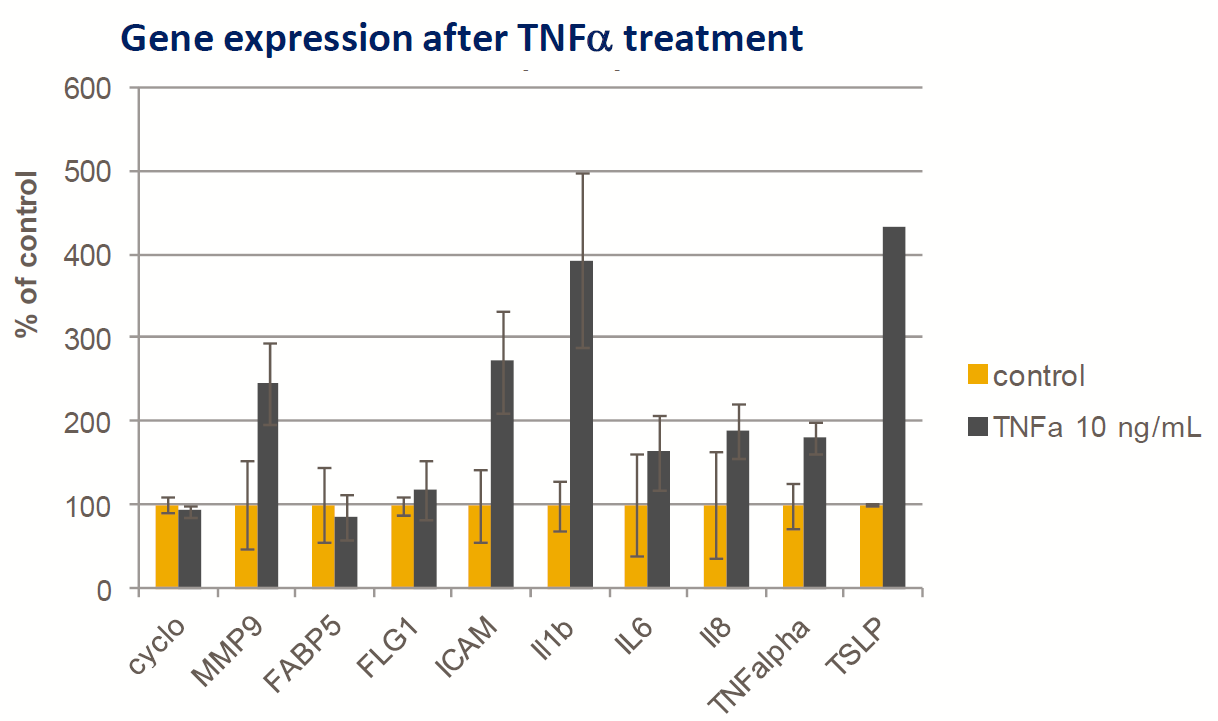
- Fresh human skin body
- TNFα (10mg/mL) added to the Franz cell lower chamber
- Induction of mild inflammatory gene response (Taqman qPCR) after 24h
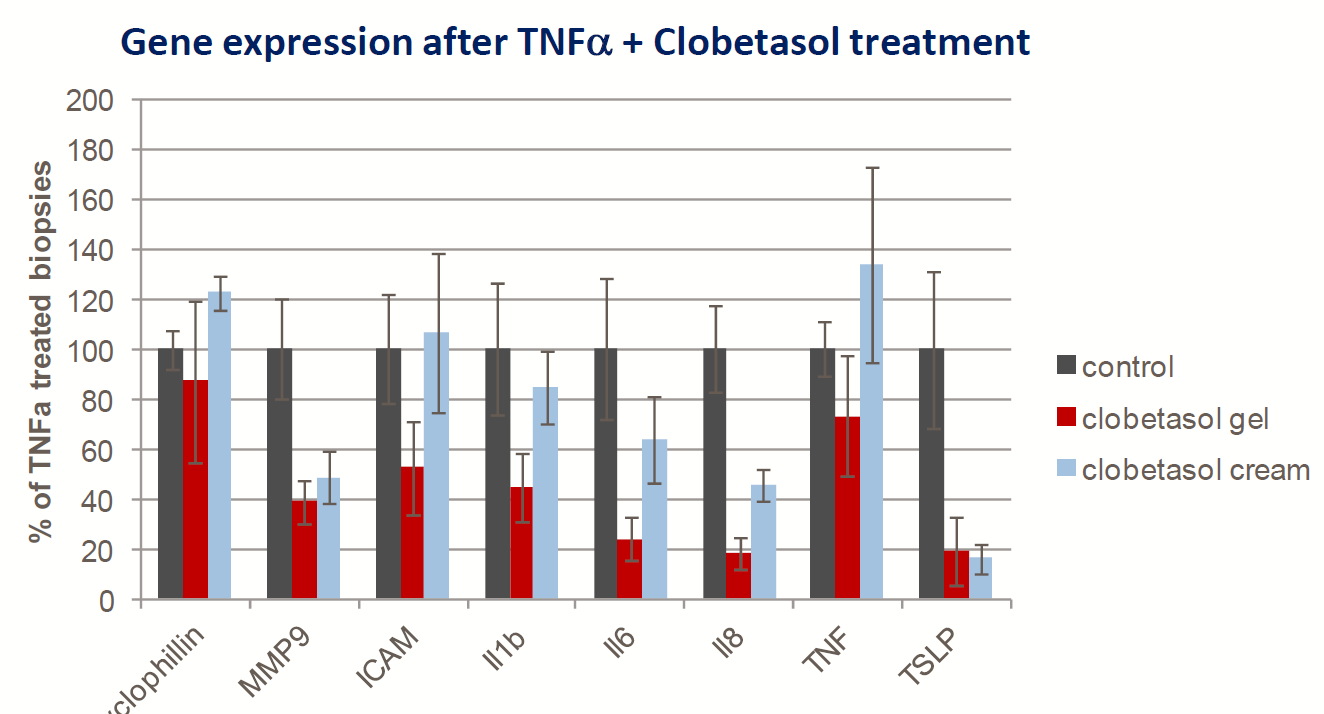
- Topical Clobetasol (corticosteroid) treatment is able to downregulate TNFα -induced gene expression (gel>cream)
Case studies for skin inflammation
-
#1 : Acute Itch
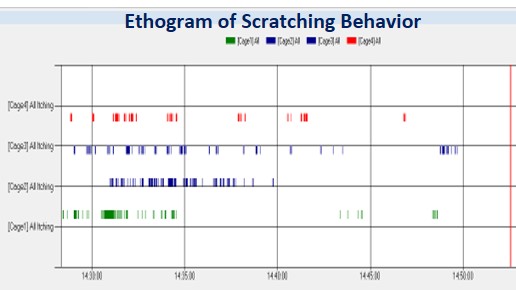
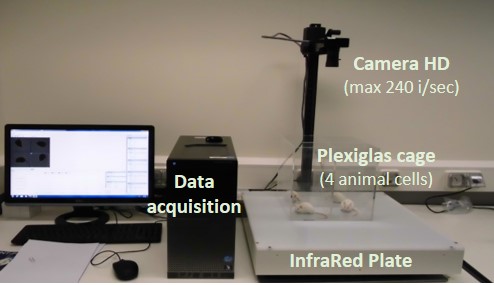
Real time scratching monitoring in mice
The neuronal pathway leading to the generation of a scratching signal is different from the pain transmission pathway.
CRO Services for acute itch models are based on intra-dermal inoculation of a pruritogen agent:
- Chloroquine
- Substance P
- (alternatively, topical Imiquimod can generate itch)
The response typically has a very fast onset & transient (<60min)
Data acquisition platform used for recording os cratching events (numbers and duration)
Evaluation of test compounds on itch relief :
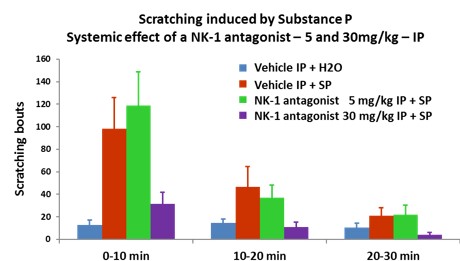
- Test compounds are applied topically 3 hours before itch induction.
- The evaluation of NK-1 antagonist is in the substance P-introduced itch model
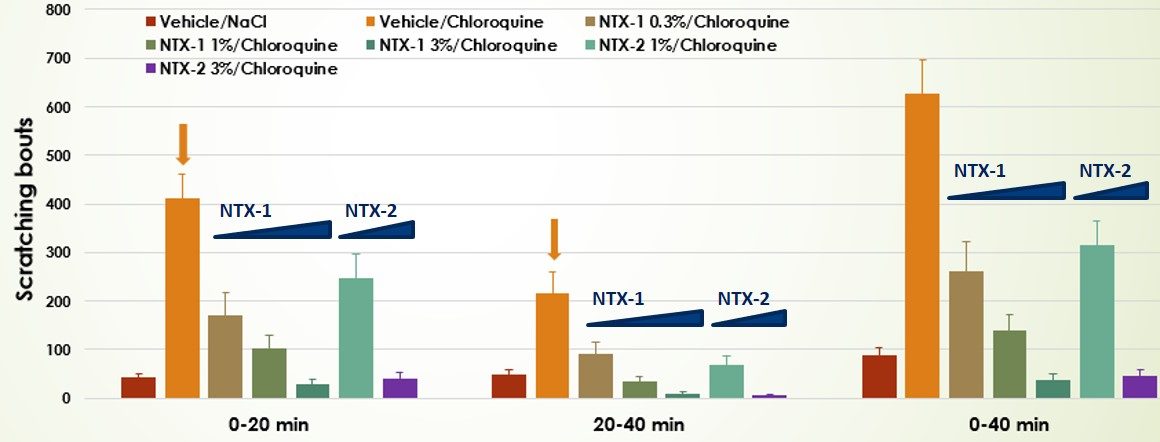
- Dose-response valuation of two test compounds in the Chloroquine-induced itch model
-
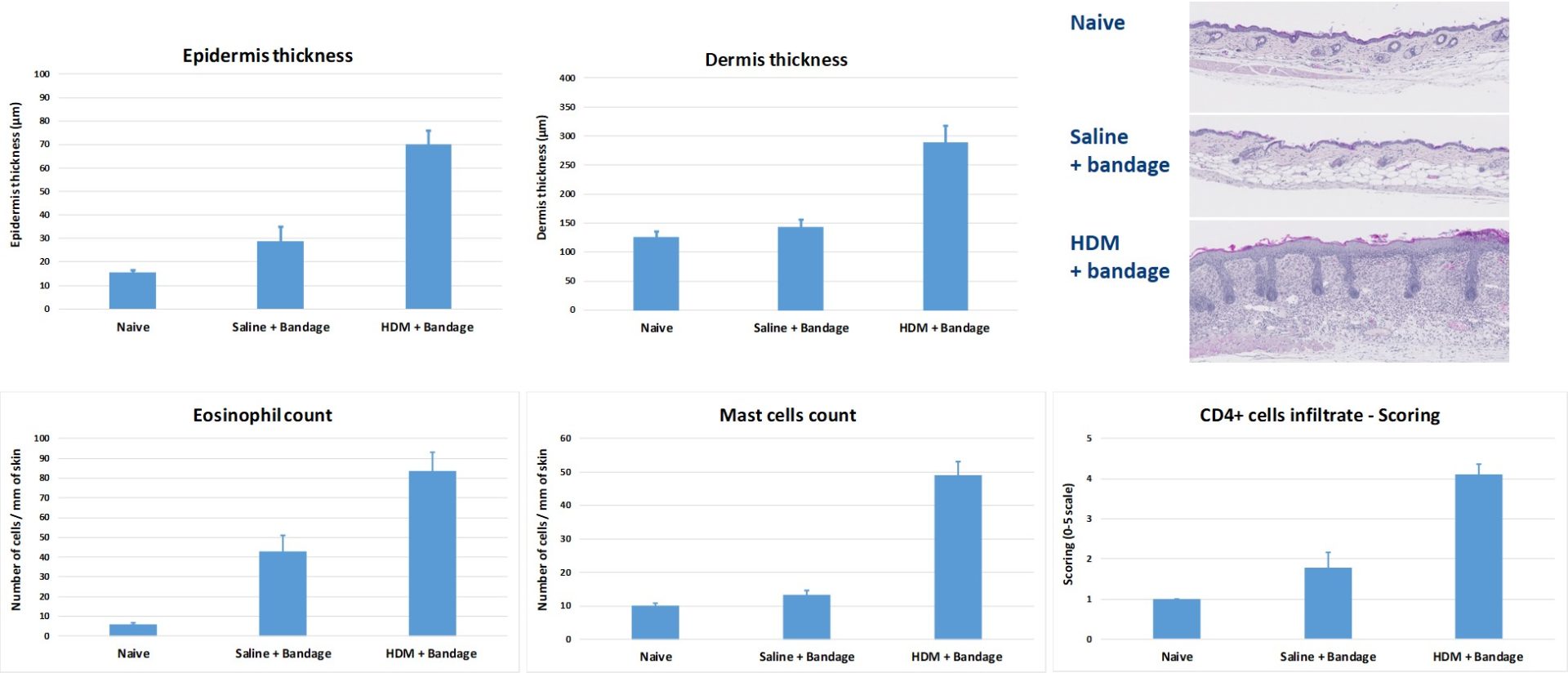
#2 : House Dust Mite (HDM)-Atopic Dermatitis (AD) mouse model
Topical application of HDM to BALB/c mice induces epidermis and dermis thickening & recruitment of inflammatory cells (eosinophils, mast cells and CD4+ cells).
This model is useful for testing the efficacy of compounds targeting general skin inflammation. HDM are applied directly on skin and the area is bandaged. The bandage is regularly replaced over the course of several weeks.
-
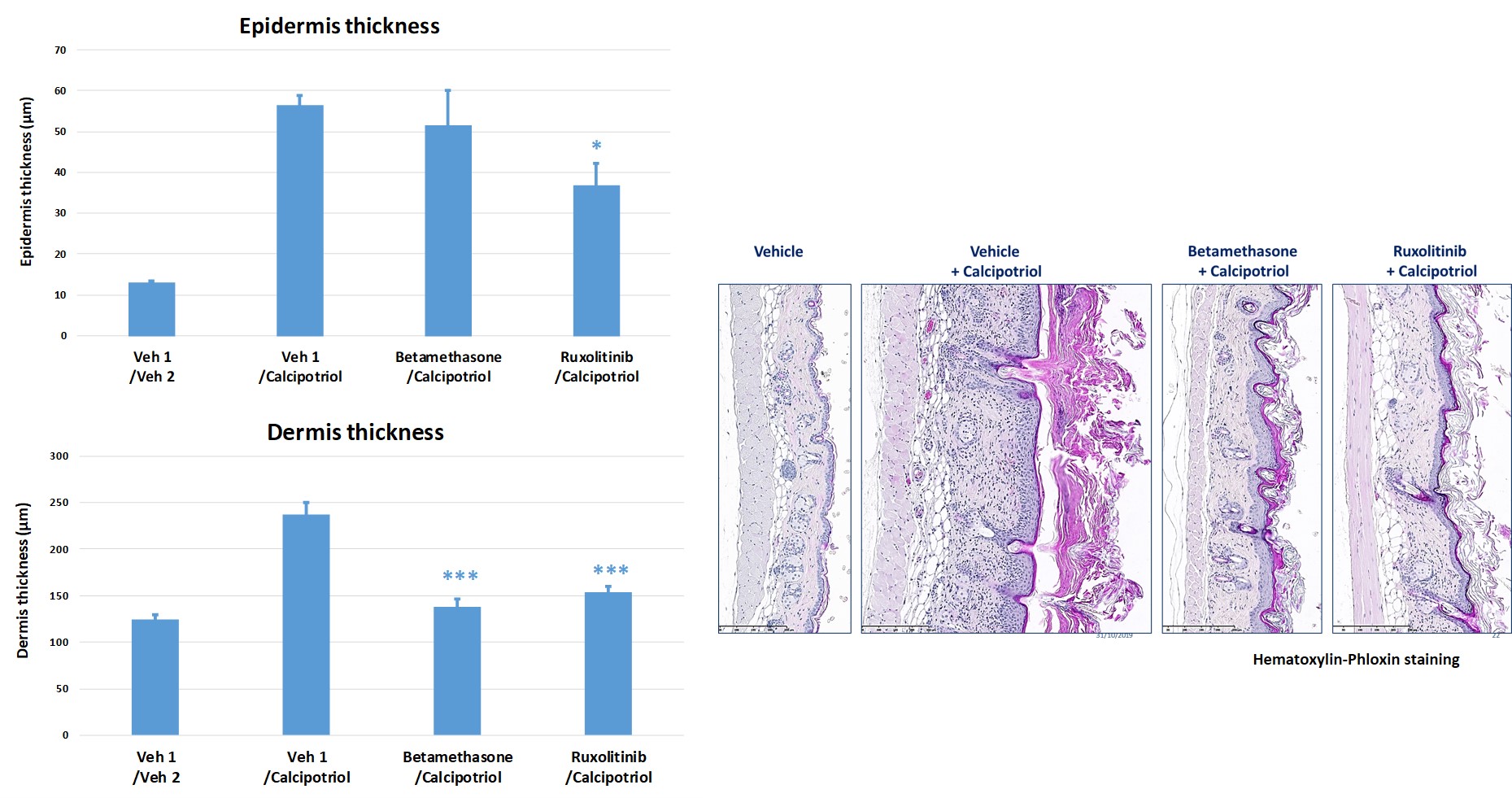
#3: Calcipotriol-induced atopic dermatitis in mice
Topical application of Calcipotriol to BALB/c mice (BID from D0 to D9) induces changes in skin morphology and inflammation resembling immune perturbations observed in acute lesions of atopic dermatitis in patients.
Topical drugs showed protective effects on skin clinical score by reducing epidermis and dermis hyperplasia.
- Betamethasone (steroid anti-inflammatory drug)
- Ruxolinitib (JAK1/JAK2 inhibitor)
-
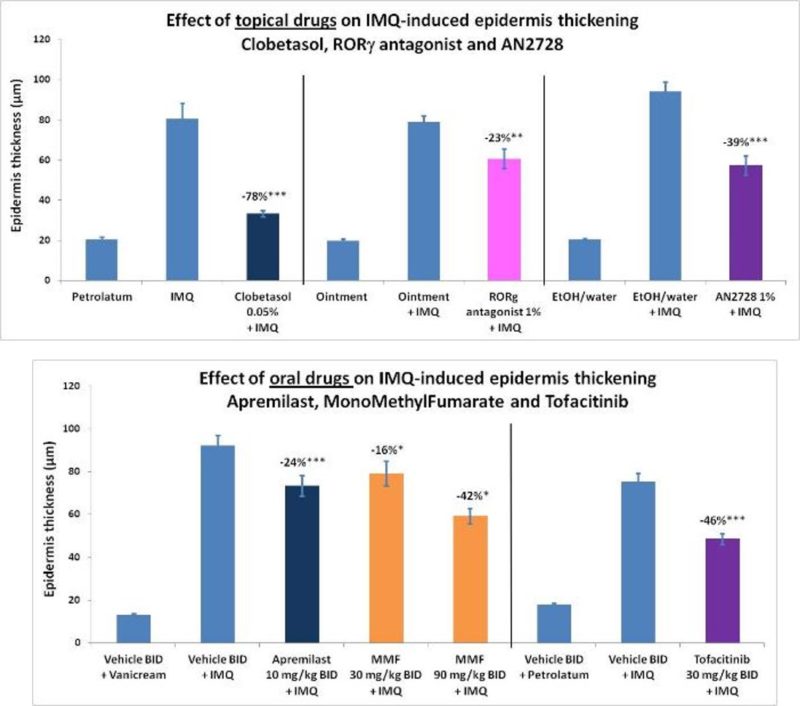
#4 : TH17 cell-targeting drugs reduce psoriasis-related inflammation
Psoriasis can result as dysregulation of the TH17 pathway:
- TH1 to TH17 (10) cell differentiation critically depends on the RORgt transcription factor
- Phosphodiesterase-4 (PDE4) is critical for the release of IL-12, IL-23 and TNFa
- Test compounds acting on RORgt (antagonist) and PDE4 (inhibitor) have demonstrated efficacy in psoriasis
Psoriasis models consistently respond to oral and topical drugs showing clinical efficacy in psoriatic patients:
- Oral drugs: Apremilast (PDE4 inhibitor), Monomethyl Fumarate (NFE2L2 inhibitor), Tofacitinib (JAK inhibitor)
- Topical drugs: Clobetasol (corticosteroid), RORgt antagonist, AN2728 (PDE4 inhibitor)
-
References
(1) Psoriasis model in mice, induced by Imiquimod
- Review:
Gangwar RS, Gudjonsson JE, Ward NL. Mouse Models of Psoriasis: A Comprehensive Review. J Invest Dermatol. 2022 Mar;142(3 Pt B):884-897. doi: 10.1016/j.jid.2021.06.019. Epub 2021 Dec 23. PMID: 34953514. : https://www.jidonline.org/article/S0022-202X(21)01442-1/fulltext
Nițescu DA, Mușetescu A, Nițescu M, Costescu M, Coman OA. Experimental research in topical psoriasis therapy (Review). Exp Ther Med. 2021 Sep;22(3):971. doi: 10.3892/etm.2021.10403. Epub 2021 Jul 8. PMID: 34335913; PMCID: PMC8290406.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8290406/
- Methods:
Singh TP, Zhang HH, Hwang ST, Farber JM. IL-23- and Imiquimod-Induced Models of Experimental Psoriasis in Mice. Curr Protoc Immunol. 2019 Jun;125(1):e71. doi: 10.1002/cpim.71. Epub 2019 Jan 7. PMID: 30615272. https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpim.71
- FACS in this model (link to FACS resources):
Lou F, Sun Y, Wang H. Protocol for Flow Cytometric Detection of Immune Cell Infiltration in the Epidermis and Dermis of a Psoriasis Mouse Model. STAR Protoc. 2020 Sep 17;1(3):100115. doi: 10.1016/j.xpro.2020.100115. PMID: 33377011; PMCID: PMC7757015. https://www.sciencedirect.com/science/article/pii/S2666166720301027?via%3Dihub
Goldstein JD, Bassoy EY, Caruso A, Palomo J, Rodriguez E, Lemeille S, Gabay C. IL-36 signaling in keratinocytes controls early IL-23 production in psoriasis-like dermatitis. Life Sci Alliance. 2020 Apr 28;3(6):e202000688. doi: 10.26508/lsa.202000688. PMID: 32345660; PMCID: PMC7190273. https://www.life-science-alliance.org/content/3/6/e202000688
- Role of immune system:
Hou Y, Zhu L, Tian H, Sun HX, Wang R, Zhang L, Zhao Y. IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell. 2018 Dec;9(12):1027-1038. doi: 10.1007/s13238-018-0505-z. Epub 2018 Mar 5. PMID: 29508278; PMCID: PMC6251802. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6251802/
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009 May 1;182(9):5836-45. doi: 10.4049/jimmunol.0802999. PMID: 19380832. https://journals.aai.org/jimmunol/article/182/9/5836/104045/Imiquimod-Induced-Psoriasis-Like-Skin-Inflammation
Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-Induced Psoriasis in Mice Depends on the IL-17 Signaling of Keratinocytes. J Invest Dermatol. 2019 May;139(5):1110-1117. doi: 10.1016/j.jid.2019.01.006. Epub 2019 Jan 23. PMID: 30684554. https://www.jidonline.org/article/S0022-202X(19)30022-3/fulltext
- Testing of compounds:
Zhang M, Li N, Cai R, Gu J, Xie F, Wei H, Lu C, Wu D. Rosmarinic acid protects mice from imiquimod induced psoriasis-like skin lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother Res. 2021 Aug;35(8):4526-4537. doi: 10.1002/ptr.7155. Epub 2021 May 18. PMID: 34008239. https://onlinelibrary.wiley.com/doi/10.1002/ptr.7155
Gao J, Chen F, Fang H, Mi J, Qi Q, Yang M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. 2020 Oct 20;53(1):48. doi: 10.1186/s40659-020-00316-0. PMID: 33081840; PMCID: PMC7576854. https://biolres.biomedcentral.com/articles/10.1186/s40659-020-00316-0
Zhang B, Lai RC, Sim WK, Choo ABH, Lane EB, Lim SK. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int J Mol Sci. 2021 Jan 13;22(2):720. doi: 10.3390/ijms22020720. PMID: 33450859; PMCID: PMC7828312. https://www.mdpi.com/1422-0067/22/2/720
Li X, Xi B, Miao Y, Ma X, Zhang J, Gao J, Wei W, Zhou H, Yang C. Nintedanib ameliorates imiquimod-induced psoriasis in mice by inhibiting NF-κB and VEGFR2 signaling. Int Immunopharmacol. 2021 Nov;100:108129. doi: 10.1016/j.intimp.2021.108129. Epub 2021 Sep 20. PMID: 34547680. https://www.sciencedirect.com/science/article/pii/S1567576921007657?via%3Dihub
Kim N, Lee S, Kang J, Choi YA, Jang YH, Jeong GS, Kim SH. Cudraxanthone D Ameliorates Psoriasis-like Skin Inflammation in an Imiquimod-Induced Mouse Model via Inhibiting the Inflammatory Signaling Pathways. Molecules. 2021 Oct 8;26(19):6086. doi: 10.3390/molecules26196086. PMID: 34641629; PMCID: PMC8512696. https://www.mdpi.com/1420-3049/26/19/6086
(2) Psoriasis model in rats, induced by Imiquimod
- Review:
Nițescu DA, Mușetescu A, Nițescu M, Costescu M, Coman OA. Experimental research in topical psoriasis therapy (Review). Exp Ther Med. 2021 Sep;22(3):971. doi: 10.3892/etm.2021.10403. Epub 2021 Jul 8. PMID: 34335913; PMCID: PMC8290406. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8290406/
- Model characterization:
Smajlović A, Haverić A, Alić A, Hadžić M, Smajlović A, Mujezinović I, Lojo-Kadrić N, Ramić J, Elez-Burnjaković N, Haverić S, Pojskić L. Molecular and histopathological profiling of imiquimod induced dermatosis in Swiss Wistar rats: contribution to the rat model for novel anti-psoriasis treatments. Mol Biol Rep. 2021 May;48(5):4295-4303. doi: 10.1007/s11033-021-06445-3. Epub 2021 Jun 7. PMID: 34097205. https://link.springer.com/article/10.1007/s11033-021-06445-3
(3) Skin scleroderma/fibrosis model in mice, induced by Bleomycin
- Review:
Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006 Feb;297(8):333-44. doi: 10.1007/s00403-005-0635-z. Epub 2006 Jan 10. PMID: 16402183. https://link.springer.com/article/10.1007/s00403-005-0635-z
Rius Rigau A, Luber M, Distler JHW. Mouse Models of Skin Fibrosis. Methods Mol Biol. 2021;2299:371-383. doi: 10.1007/978-1-0716-1382-5_25. PMID: 34028755. https://link.springer.com/protocol/10.1007/978-1-0716-1382-5_25
- Methods:
Błyszczuk P, Kozlova A, Guo Z, Kania G, Distler O. Experimental Mouse Model of Bleomycin-Induced Skin Fibrosis. Curr Protoc Immunol. 2019 Sep;126(1):e88. doi: 10.1002/cpim.88. PMID: 31483105. https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpim.88
- Involvement of the immune system:
Park MJ, Moon SJ, Lee EJ, Jung KA, Kim EK, Kim DS, Lee JH, Kwok SK, Min JK, Park SH, Cho ML. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front Immunol. 2018 Jul 10;9:1611. doi: 10.3389/fimmu.2018.01611. PMID: 30042768; PMCID: PMC6048384. https://www.frontiersin.org/articles/10.3389/fimmu.2018.01611/full
Liu S, Herault Y, Pavlovic G, Leask A. Skin progenitor cells contribute to bleomycin-induced skin fibrosis. Arthritis Rheumatol. 2014 Mar;66(3):707-13. doi: 10.1002/art.38276. PMID: 24574231. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.38276
Shou Y, Yang L, Yang Y, Xu J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021 Oct 27;12(11):1009. doi: 10.1038/s41419-021-04284-5. PMID: 34707088; PMCID: PMC8551323. https://www.nature.com/articles/s41419-021-04284-5
- Testing of treatments:
Moon J, Lee SY, Choi JW, Lee AR, Yoo JH, Moon SJ, Park SH, Cho ML. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J Transl Med. 2021 May 4;19(1):192. doi: 10.1186/s12967-021-02860-z. Erratum in: J Transl Med. 2021 Jun 21;19(1):266. PMID: 33947424; PMCID: PMC8097822. https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-021-02860-z
Li R, Yin H, Wang J, He D, Yan Q, Lu L. Dihydroartemisinin alleviates skin fibrosis and endothelial dysfunction in bleomycin-induced skin fibrosis models. Clin Rheumatol. 2021 Oct;40(10):4269-4277. doi: 10.1007/s10067-021-05765-w. Epub 2021 May 19. PMID: 34013490. https://link.springer.com/article/10.1007/s10067-021-05765-w
(4) Skin scleroderma/fibrosis model in mice, induced by Topoisomerase-I peptide-loaded dendritic cells
- Review:
Rius Rigau A, Luber M, Distler JHW. Mouse Models of Skin Fibrosis. Methods Mol Biol. 2021;2299:371-383. doi: 10.1007/978-1-0716-1382-5_25. PMID: 34028755. https://link.springer.com/protocol/10.1007/978-1-0716-1382-5_25
- Model characterization:
Mehta H, Goulet PO, Nguyen V, Pérez G, Koenig M, Senécal JL, Sarfati M. Topoisomerase I peptide-loaded dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity. 2016 Dec;49(8):503-513. doi: 10.1080/08916934.2016.1230848. Epub 2016 Nov 3. PMID: 27808577. https://www.tandfonline.com/doi/abs/10.1080/08916934.2016.1230848?journalCode=iaut20
- Role of microbiome
Mehta H, Goulet PO, Mashiko S, Desjardins J, Pérez G, Koenig M, Senécal JL, Constante M, Santos MM, Sarfati M. Early-Life Antibiotic Exposure Causes Intestinal Dysbiosis and Exacerbates Skin and Lung Pathology in Experimental Systemic Sclerosis. J Invest Dermatol. 2017 Nov;137(11):2316-2325. doi: 10.1016/j.jid.2017.06.019. Epub 2017 Jul 27. PMID: 28757138. https://www.jidonline.org/article/S0022-202X(17)31854-7/fulltext
Ho KJ, Varga J. Early-Life Gut Dysbiosis: A Driver of Later-Life Fibrosis? J Invest Dermatol. 2017 Nov;137(11):2253-2255. doi: 10.1016/j.jid.2017.08.017. PMID: 29055411. https://www.jidonline.org/article/S0022-202X(17)32831-2/fulltext
(5) Skin scleroderma/fibrosis model in mice, induced by Topoisomerase-I and CFA
- Review:
Rius Rigau A, Luber M, Distler JHW. Mouse Models of Skin Fibrosis. Methods Mol Biol. 2021;2299:371-383. doi: 10.1007/978-1-0716-1382-5_25. PMID: 34028755. https://link.springer.com/protocol/10.1007/978-1-0716-1382-5_25
Yoshizaki A, Yanaba K, Ogawa A, Asano Y, Kadono T, Sato S. Immunization with DNA topoisomerase I and Freund’s complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011 Nov;63(11):3575-85. doi: 10.1002/art.30539. PMID: 21792823. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.30539
(6) Atopic dermatitis model in mice, induced by Calcipotriol
- Reviews:
Guerrero-Aspizua S, Carretero M, Conti CJ, Del Río M. The importance of immunity in the development of reliable animal models for psoriasis and atopic dermatitis. Immunol Cell Biol. 2020 Sep;98(8):626-638. doi: 10.1111/imcb.12365. Epub 2020 Jul 15. PMID: 32479655. https://onlinelibrary.wiley.com/doi/10.1111/imcb.12365
Kim D, Kobayashi T, Nagao K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J Invest Dermatol. 2019 May;139(5):984-990.e1. doi: 10.1016/j.jid.2019.02.014. Epub 2019 Apr 19. PMID: 31010529; PMCID: PMC6555635. https://www.jidonline.org/article/S0022-202X(19)30184-8/fulltext
- Methods:
Moosbrugger-Martinz V, Schmuth M, Dubrac S. A Mouse Model for Atopic Dermatitis Using Topical Application of Vitamin D3 or of Its Analog MC903. Methods Mol Biol. 2017;1559:91-106. doi: 10.1007/978-1-4939-6786-5_8. PMID: 28063040. https://link.springer.com/protocol/10.1007/978-1-4939-6786-5_8
- Immune mechanisms:
Wan H, Yang H, Wei M, Chen W. Polyinosinic:polycytidylic acid aggravates calcipotriol-induced atopic dermatitis-like skin lesions in mice by increasing the expression of thymic stromal lymphopoietin. Ann Transl Med. 2022 Feb;10(4):209. doi: 10.21037/atm-22-282. PMID: 35280398; PMCID: PMC8908153. https://atm.amegroups.com/article/view/90304/html
Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11736-41. doi: 10.1073/pnas.0604575103. Epub 2006 Jul 31. PMID: 16880407; PMCID: PMC1544239. https://www.pnas.org/doi/10.1073/pnas.0604575103?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed
Li M, Hener P, Zhang Z, Ganti KP, Metzger D, Chambon P. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J Invest Dermatol. 2009 Feb;129(2):498-502. doi: 10.1038/jid.2008.232. Epub 2008 Jul 24. PMID: 18650845. https://www.jidonline.org/article/S0022-202X(15)34183-X/fulltext
- Compound testing :
Seshimo H, Egusa C, Maeda T, Numata T, Okubo Y, Harada K, Ito T. Topical application of imatinib mesylate suppresses vitamin D3 analog-induced dermatitis in Balb/c mice. Exp Dermatol. 2022 Dec 1. doi: 10.1111/exd.14720. Epub ahead of print. PMID: 36457228. https://onlinelibrary.wiley.com/doi/10.1111/exd.14720
(7) Atopic dermatitis model in mice, induced by DNFB
- Reviews:
Guerrero-Aspizua S, Carretero M, Conti CJ, Del Río M. The importance of immunity in the development of reliable animal models for psoriasis and atopic dermatitis. Immunol Cell Biol. 2020 Sep;98(8):626-638. doi: 10.1111/imcb.12365. Epub 2020 Jul 15. PMID: 32479655. https://onlinelibrary.wiley.com/doi/10.1111/imcb.12365
Kim D, Kobayashi T, Nagao K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J Invest Dermatol. 2019 May;139(5):984-990.e1. doi: 10.1016/j.jid.2019.02.014. Epub 2019 Apr 19. PMID: 31010529; PMCID: PMC6555635. https://www.jidonline.org/article/S0022-202X(19)30184-8/fulltext
- Testing of compounds:
Tang L, Gao J, Cao X, Chen L, Wang H, Ding H. TRPV1 mediates itch-associated scratching and skin barrier dysfunction in DNFB-induced atopic dermatitis mice. Exp Dermatol. 2022 Mar;31(3):398-405. doi: 10.1111/exd.14464. Epub 2021 Oct 11. PMID: 34608683. https://onlinelibrary.wiley.com/doi/10.1111/exd.14464
Liu Q, Li M, Wang N, He C, Jiang X, Li J. Calcium-Based Antimicrobial Peptide Compounds Attenuate DNFB-Induced Atopic Dermatitis-Like Skin Lesions via Th-Cells in BALB/c Mice. Int J Mol Sci. 2022 Sep 26;23(19):11371. doi: 10.3390/ijms231911371. PMID: 36232673; PMCID: PMC9569644. https://www.mdpi.com/1422-0067/23/19/11371
Gao JF, Tang L, Luo F, Zhang YY, Chen L, Ding H, Meng ZD. Nicotinamide mononucleotide ameliorates DNFB-induced atopic dermatitis-like symptoms in mice by blocking activation of ROS-mediated JAK2/STAT5 signaling pathway. Int Immunopharmacol. 2022 Aug;109:108812. doi: 10.1016/j.intimp.2022.108812. Epub 2022 May 6. PMID: 35533554. https://www.sciencedirect.com/science/article/pii/S156757692200296X?via%3Dihub
(8) Atopic dermatitis model in mice, induced by HDM
- Reviews:
Guerrero-Aspizua S, Carretero M, Conti CJ, Del Río M. The importance of immunity in the development of reliable animal models for psoriasis and atopic dermatitis. Immunol Cell Biol. 2020 Sep;98(8):626-638. doi: 10.1111/imcb.12365. Epub 2020 Jul 15. PMID: 32479655 https://onlinelibrary.wiley.com/doi/10.1111/imcb.12365
Kim D, Kobayashi T, Nagao K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J Invest Dermatol. 2019 May;139(5):984-990.e1. doi: 10.1016/j.jid.2019.02.014. Epub 2019 Apr 19. PMID: 31010529; PMCID: PMC6555635. https://www.jidonline.org/article/S0022-202X(19)30184-8/fulltext
- Mechanism:
Lee YS, Choi JH, Lee JH, Lee HW, Lee W, Kim WT, Kim TY. Extracellular superoxide dismutase ameliorates house dust mite-induced atopic dermatitis-like skin inflammation and inhibits mast cell activation in mice. Exp Dermatol. 2016 Aug;25(8):630-5. doi: 10.1111/exd.13028. Epub 2016 Jun 30. PMID: 27061078. https://onlinelibrary.wiley.com/doi/10.1111/exd.13028
(9) Pruritogen itch model in mice, induced by Chloroquine, Substance P or Imiquimod
- Scratching measurement and comparison of mouse strains:
Sargent JL, Löhr CV, Diggs HE. Scratching Responses to Epidermal Injury in C57BL/6, DBA/2, BALB/c, and CD1 Mice. Comp Med. 2016;66(3):208-15. PMID: 27298245; PMCID: PMC4907529. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4907529/
- Chloroquine:
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009 Dec 24;139(7):1353-65. doi: 10.1016/j.cell.2009.11.034. Epub 2009 Dec 10. PMID: 20004959; PMCID: PMC2989405. https://www.cell.com/cell/fulltext/S0092-8674(09)01492-5
Shiraishi Y, Koga K, Yamagata R, Hatada I, Shiratori-Hayashi M, Tsuda M. α1A-adrenaline receptors in dorsal horn inhibitory neurons have an inhibitory role in the regulation of chloroquine-induced itch in mice. Mol Brain. 2021 Mar 16;14(1):55. doi: 10.1186/s13041-021-00768-9. PMID: 33726812; PMCID: PMC7962300. https://molecularbrain.biomedcentral.com/articles/10.1186/s13041-021-00768-9
- Chloroquine itch and microbiota:
Zhang Q, Li T, Niu J, Xiao J, Zhang M, Zhang R, Chen D, Shi Y, Zhang X, Hu X, Yu B, Feng J, Fang Q. Inhibitory effects of antibiotic-induced gut microbiota depletion on acute itch behavior in mice. Brain Res Bull. 2022 Nov;190:50-61. doi: 10.1016/j.brainresbull.2022.09.014. Epub 2022 Sep 17. PMID: 36126873. https://www.sciencedirect.com/science/article/abs/pii/S0361923022002544?via%3Dihub
Li R, Sun H, Zheng H, Zong Z, Li S, Meng T, Li J, Liu Y, Wang C, Li J. Intradermal Injection of Oxytocin Aggravates Chloroquine-Induced Itch Responses via Activating the Vasopressin-1a Receptor/Nitric Oxide Pathway in Mice. Front Pharmacol. 2019 Nov 15;10:1380. doi: 10.3389/fphar.2019.01380. PMID: 31824317; PMCID: PMC6881818. https://www.frontiersin.org/articles/10.3389/fphar.2019.01380/full
- Substance P:
Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001 Dec;117(6):1621-6. doi: 10.1046/j.0022-202x.2001.01585.x. PMID: 11886531. https://www.jidonline.org/article/S0022-202X(15)41505-2/fulltext
Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol. 2017 Aug;140(2):447-453.e3. doi: 10.1016/j.jaci.2016.12.980. Epub 2017 Feb 20. PMID: 28219706; PMCID: PMC5546940. https://www.jacionline.org/article/S0091-6749(17)30230-0/fulltext
Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. Br J Pharmacol. 2003 Jan;138(1):202-8. doi: 10.1038/sj.bjp.0705004. PMID: 12522091; PMCID: PMC1573631. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/sj.bjp.0705004
Akasaka Y, Yoshida T, Tsukahara M, Hatta A, Inoue H. Glycyrrhetinic acid prevents cutaneous scratching behavior in mice elicited by substance P or PAR-2 agonist. Eur J Pharmacol. 2011 Nov 16;670(1):175-9. doi: 10.1016/j.ejphar.2011.08.043. Epub 2011 Sep 10. PMID: 21925497. https://www.sciencedirect.com/science/article/abs/pii/S0014299911009538?via%3Dihub
Andoh T, Kuraishi Y. Inhibitory effects of azelastine on substance P-induced itch-associated response in mice. Eur J Pharmacol. 2002 Feb 2;436(3):235-9. doi: 10.1016/s0014-2999(01)01617-x. PMID: 11858803. https://www.sciencedirect.com/science/article/abs/pii/S001429990101617X?via%3Dihub
- Imiquimod-induced itch:
Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016 Nov;157(11):2536-2543. doi: 10.1097/j.pain.0000000000000674. PMID: 27437787; PMCID: PMC5069152. https://journals.lww.com/pain/Abstract/2016/11000/Mouse_model_of_imiquimod_induced_psoriatic_itch.19.aspx
Li L, Liu X, Ge W, Chen C, Huang Y, Jin Z, Zhan M, Duan X, Liu X, Kong Y, Jiang J, Li X, Zeng X, Li F, Xu S, Li M, Chen H. CB2R Deficiency Exacerbates Imiquimod-Induced Psoriasiform Dermatitis and Itch Through the Neuro-Immune Pathway. Front Pharmacol. 2022 Jan 31;13:790712. doi: 10.3389/fphar.2022.790712. PMID: 35173615; PMCID: PMC8841964. https://www.frontiersin.org/articles/10.3389/fphar.2022.790712/full
Xu Z, Qin Z, Zhang J, Wang Y. Microglia-mediated chronic psoriatic itch induced by imiquimod. Mol Pain. 2020 Jan-Dec;16:1744806920934998. doi: 10.1177/1744806920934998. PMID: 32580615; PMCID: PMC7318815. https://journals.sagepub.com/doi/full/10.1177/1744806920934998
(10) Acnea model in rats and mice, induced by Sebaceous gland atrophy
Meingassner JG, Aschauer H, Winiski AP, Dales N, Yowe D, Winther MD, Zhang Z, Stütz A, Billich A. Pharmacological inhibition of stearoyl CoA desaturase in the skin induces atrophy of the sebaceous glands. J Invest Dermatol. 2013 Aug;133(8):2091-4. doi: 10.1038/jid.2013.89. Epub 2013 Feb 27. PMID: 23446987. https://www.jidonline.org/article/S0022-202X(15)36362-4/fulltext
Shang W, Tan AYQ, van Steensel MAM, Lim X. Aberrant Wnt Signaling Induces Comedo-Like Changes in the Murine Upper Hair Follicle. J Invest Dermatol. 2022 Oct;142(10):2603-2612.e6. doi: 10.1016/j.jid.2021.11.034. Epub 2021 Dec 17. PMID: 34929175. https://www.jidonline.org/article/S0022-202X(21)02616-6/fulltext
Case studies for skin inflammation:
- #1 : Acute Itch : Same refs as above
- #2 : House Dust Mite (HDM)-Atopic Dermatitis (AD) mouse model : Same refs as above
- #3: Calcipotriol-induced atopic dermatitis in mice : Same refs as above
- #4: TH17 cell-targeting drugs reduce psoriasis-related inflammation
Zhang M, Li N, Cai R, Gu J, Xie F, Wei H, Lu C, Wu D. Rosmarinic acid protects mice from imiquimod induced psoriasis-like skin lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother Res. 2021 Aug;35(8):4526-4537. doi: 10.1002/ptr.7155. Epub 2021 May 18. PMID: 34008239. https://onlinelibrary.wiley.com/doi/10.1002/ptr.7155
Shou Y, Yang L, Yang Y, Xu J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021 Oct 27;12(11):1009. doi: 10.1038/s41419-021-04284-5. PMID: 34707088; PMCID: PMC8551323. https://www.nature.com/articles/s41419-021-04284-5
