Preclinical CRO Services for the Study of Systemic Inflammation
Systemic inflammatory diseases are triggered by dysregulated activation of immune cells. It can be frequently observed in a variety of organ transplantation cases or septic shock. Rodent model studies are helpful to decipher the disease triggering or sustaining mechanisms observed in patients.
Oncodesign Services offers access several preclinical models addressing a variety of systemic inflammation mechanisms, and provides support to the development of research models meeting your specific needs.
Typical readouts for systemic inflammation
- Clinical scoring
- Body weight
- Body temperature (for shock models)
- Survival
- Histopathology
- Biomarker/drug monitoring
- Gene expression in relevant tissues, by qPCR/dPCR
- Immune phenotyping
Discover Oncodesign Services offers for systemic inflammation diseases
-
In vitro assays
- LPS or TNFa stimulation of human primary monocytes
- TLR ligand stimulation on human PBMC and human whole blood
- TLR ligand stimulation on mouse RAW264.7 macrophage cell line
-
Systemic Inflammation models List
- Graft-versus-host disease (1), allogeneic, with Balb/c (H2d) irradiated mice or with C57b6D2F1 (H2d) mice.
- Graft-versus-host disease, xenogeneic, in NOG mice.
- Systemic inflammatory response syndrome (2), induced by TNFa, causing hypothermia.
- Systemic IFNg response (3), IL-12/IL-18-induced.

Case studies
-
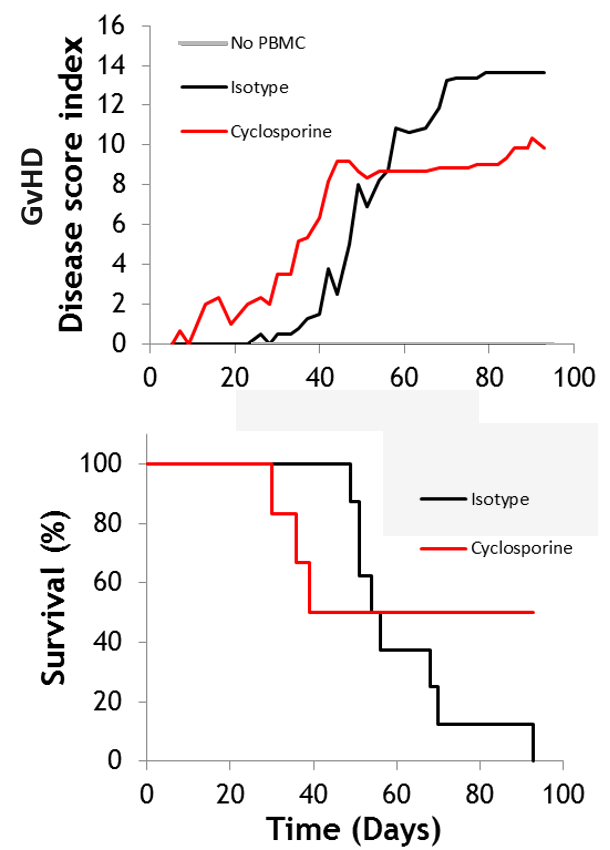
#1 Xenogeneic GvHD
Graft-versus host disease (GvHD) is a major inflammatory complication after hematopoietic cell transplantation, with around 50% of patients undergoing GvHD. Xenogeneic GvHD models use human donor cells transplanted into NOG immuno-deficient mice, with monitoring over 30+ days. Cyclosporine is typically used as a reference compound.
-
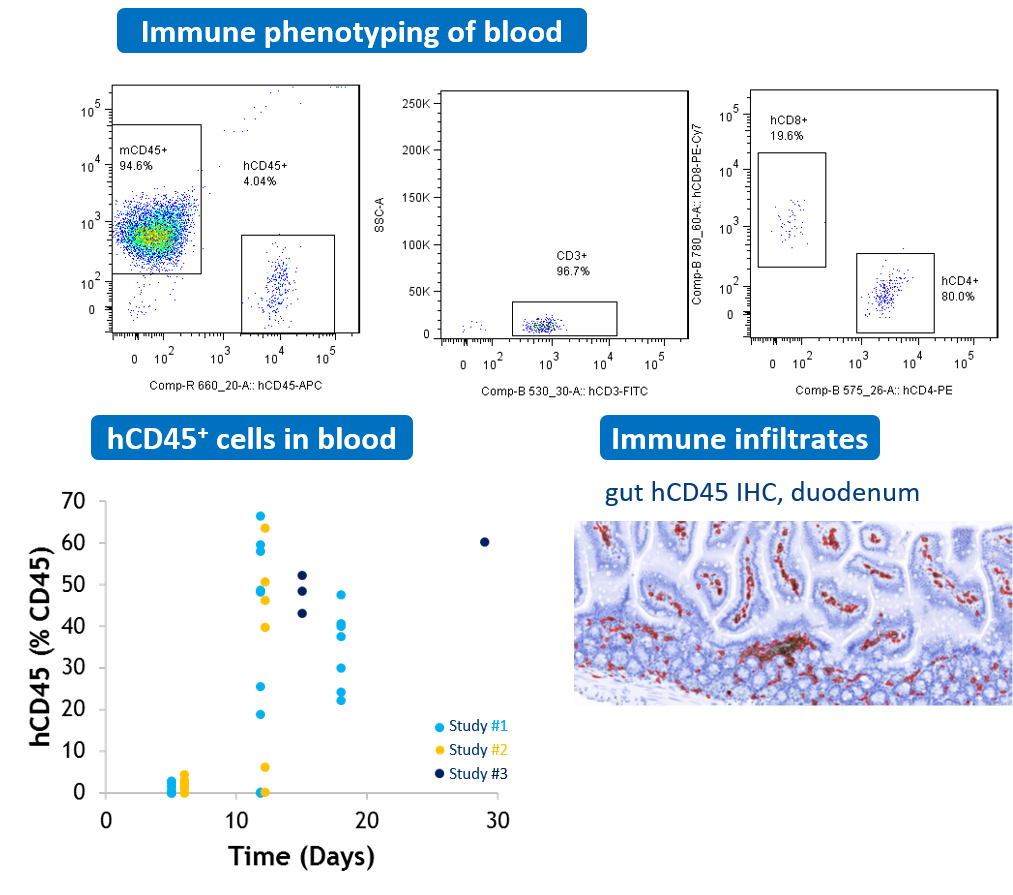
#2 GVHD xenogeneic mouse model
NOG mice are transplanted IP with human PBMCs. The analysis of human T cell population performed by flow cytometry is used for longitudinal monitoring of human (hCD45+) leucocytes frequency in blood. Human immune infiltrates are also observed in tissues such as gastro-intestinal tract.
Also to discover
-
References
(1) Graft-versus-host disease:
Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, Eto T, Ito M. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009 Jun 15;87(11):1654-8. doi: 10.1097/TP.0b013e3181a5cb07. PMID: 19502956.
https://pubmed.ncbi.nlm.nih.gov/19502956/
Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011 May;4(3):318-33. doi: 10.1242/dmm.006668. PMID: 21558065; PMCID: PMC3097454.
https://pubmed.ncbi.nlm.nih.gov/21558065/
Ye C, Yang H, Cheng M, Shultz LD, Greiner DL, Brehm MA, Keck JG. A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. FASEB J. 2020 Sep;34(9):12963-12975. doi: 10.1096/fj.202001203R. Epub 2020 Aug 9. PMID: 32772418; PMCID: PMC7436391.
https://pubmed.ncbi.nlm.nih.gov/32772418/
Elhage A, Sligar C, Cuthbertson P, Watson D, Sluyter R. Insights into mechanisms of graft-versus-host disease through humanised mouse models. Biosci Rep. 2022 Sep 30;42(9):BSR20211986. doi: 10.1042/BSR20211986. PMID: 35993192; PMCID: PMC9446388.
https://pubmed.ncbi.nlm.nih.gov/35993192/
(2) Systemic inflammatory response syndrome, induced by TNFa, causing hypothermia.
Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011 Dec 23;35(6):908-18. doi: 10.1016/j.immuni.2011.09.020. PMID: 22195746.
https://pubmed.ncbi.nlm.nih.gov/22195746/
Harris PA, Berger SB, Jeong JU, Nagilla R, Bandyopadhyay D, Campobasso N, Capriotti CA, Cox JA, Dare L, Dong X, Eidam PM, Finger JN, Hoffman SJ, Kang J, Kasparcova V, King BW, Lehr R, Lan Y, Leister LK, Lich JD, MacDonald TT, Miller NA, Ouellette MT, Pao CS, Rahman A, Reilly MA, Rendina AR, Rivera EJ, Schaeffer MC, Sehon CA, Singhaus RR, Sun HH, Swift BA, Totoritis RD, Vossenkämper A, Ward P, Wisnoski DD, Zhang D, Marquis RW, Gough PJ, Bertin J. Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases. J Med Chem. 2017 Feb 23;60(4):1247-1261. doi: 10.1021/acs.jmedchem.6b01751. Epub 2017 Feb 10. PMID: 28151659.
https://pubmed.ncbi.nlm.nih.gov/28151659/
Zelic M, Roderick JE, O’Donnell JA, Lehman J, Lim SE, Janardhan HP, Trivedi CM, Pasparakis M, Kelliher MA. RIP kinase 1-dependent endothelial necroptosis underlies systemic inflammatory response syndrome. J Clin Invest. 2018 May 1;128(5):2064-2075. doi: 10.1172/JCI96147. Epub 2018 Apr 16. PMID: 29664014; PMCID: PMC5919800.
https://pubmed.ncbi.nlm.nih.gov/29664014/
(3) Systemic IFNg response, IL-12/IL-18-induced.
Burke JR, Cheng L, Gillooly KM, Strnad J, Zupa-Fernandez A, Catlett IM, Zhang Y, Heimrich EM, McIntyre KW, Cunningham MD, Carman JA, Zhou X, Banas D, Chaudhry C, Li S, D’Arienzo C, Chimalakonda A, Yang X, Xie JH, Pang J, Zhao Q, Rose SM, Huang J, Moslin RM, Wrobleski ST, Weinstein DS, Salter-Cid LM. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019 Jul 24;11(502):eaaw1736. doi: 10.1126/scitranslmed.aaw1736. PMID: 31341059.
https://pubmed.ncbi.nlm.nih.gov/31341059/
Nakamura S, Otani T, Ijiri Y, Motoda R, Kurimoto M, Orita K. IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J Immunol. 2000 Mar 15;164(6):3330-6. doi: 10.4049/jimmunol.164.6.3330. PMID: 10706727.
https://pubmed.ncbi.nlm.nih.gov/10706727/