

Preclinical models to evaluate new therapies for prostate cancer
Prostate cancer (PCa) is currently the second most commonly diagnosed cancer and the fifth leading cause of death among men worldwide.
Early detection followed by prostatectomy leads to a very high 15-year survival but many men globally still do not have access to routine screening leading to later-stage diagnosis. Other than surgery, common treatments include radiation, hormone blockers or chemotherapy agents such as docetaxel. Recent targeted therapy approaches such as BRCA mutant inhibitors and molecular radiotherapies targeting PSMA show promise.
Oncodesign Services provides in vivo models of prostate cancer
Oncodesign Services offers testing of therapies on CDX and PDX models of androgen-sensitive and androgen-refractory prostate cancer as subcutaneous tumor models in mice. Syngeneic models are also available for therapies requiring an intact host immune system.
Metastasis: we also offer prostate-to-bone modeling as a proxy for bone mets, a common metastasis site for stage-IV prostate cancers. Drug exposure in the bone tumor microenvironment (TME) is sufficiently different from the subcutaneous TME to warrant this advanced modeling for therapies aiming to treat metastatic disease.
For in vivo preclinical translational pharmacology and ADME/DMPK purposes these include the following human CDX (cell line-derived xenograft) and PDX (patient-derived xenograft) as well as syngeneic animal models mimicking the different clinical situation of this cancer pathology:
| Clinical features | Syngeneic models | CDX models | PDX models |
| Androgen-sensitive | R3327H | CWR22 MDA PCa2b (PSMA expressing) |
PAC-120 |
| Androgen-refractory (castration resistant, Hormone-refractory) | R3327-AT3 | DU145 PC-3M PC-3MM2 LnCap-C4.2 (PSMA expressing) CWR22-Rv1 (PSMA expressing) |
LuCAP 96CR (PSMA expressing) LuCaP 136CR |
| Advanced Metastatic disease | R3327-MatLyLu (lung mets) | PC-3 (bone mets) |
Oncodesign Services offers related services such as:
- In vitro assays
- Biodistribution
- Biomarker identification & validation
We are pleased to receive any inquiries you have about ways to develop your drug therapies for prostate cancer!
Case study :
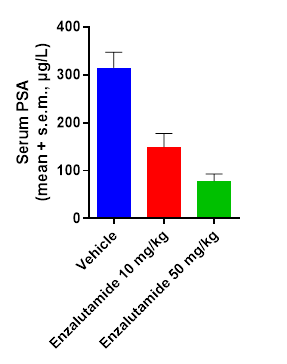
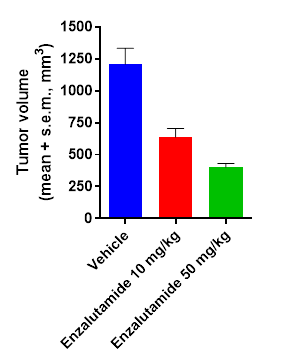
Subcutaneous LnCap C4.2 model – Tumor growth inhibition and decrease of PSA biomarker following enzalutamide treatment
Contact us for more information
