


Boost your drug development up to IND with experimented preclinical research services
Preclinical research refers to the stage of scientific investigations that occurs before a new drug or medical treatment is tested in humans through clinical trials. This phase involves extensive laboratory and in vivo studies to assess the safety, efficacy, and potential mechanisms of action of a drug candidate.
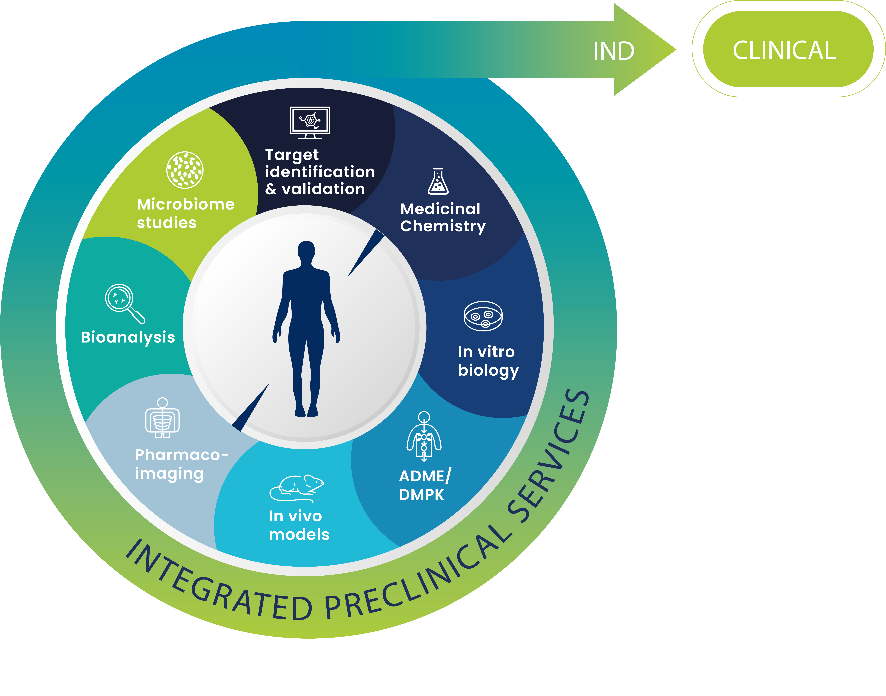
Collaborate with a CRO for your preclinical research program gives access to essential data to deliver IND.
Key aspects of Preclinical Research
With 25 years of experiences in preclinical research, Oncodesign Services gathers quality data to support the initiation of clinical trials.
The combination of translational research capabilities (medicinal chemistry, in vitro assays, DMPK/ADME, in vivo models) provide a better understanding of how drug might behave in the human body, and includes:
- Toxicity and side effects of drug candidate
- Proof of concept and efficacy studies
- Optimization of dose and formulation for optimal therapeutic effect
- Elimination of unpromising candidates early in the drug development process
This integrated approach can completely be tailored to your specific needs. Based on our extensive experience, Oncodesign Services’ team offers support along your projects with accelerated cycles times and the latest innovative technologies.

A leading CRO dedicated to preclinical research services
Our range of preclinical research services is provided across all of our sites (Dijon and Paris), for oncology, inflammation and infectious diseases services. Regardless of research program, companies can choose from services that include lead optimization and preclinical research as integrated or stand-alone programs.
Do you want to know more about our capabilities ?


